Revised_for_DT_final-1 - Pure
... Metal vinylidene complexes are widely encountered, or postulated, as intermediates in a range of important metalmediated transformations of alkynes. However, fluorovinylidene complexes have rarely been described and their reactivity is largely unexplored. By making use of the novel outer-sphere elec ...
... Metal vinylidene complexes are widely encountered, or postulated, as intermediates in a range of important metalmediated transformations of alkynes. However, fluorovinylidene complexes have rarely been described and their reactivity is largely unexplored. By making use of the novel outer-sphere elec ...
aldehydes and ketones
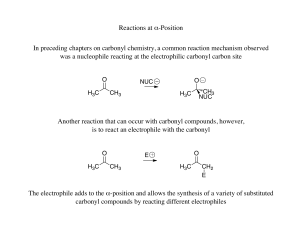
... The reactivity of the carbonyl group towards the addition reaction depends upon the magnitude of the positive charge on the carbonyl carbon atom. Hence, any substituent that increases the positive charge on the carbonyl carbon must increase its reactivity towards addition reactions. The introduction ...
... The reactivity of the carbonyl group towards the addition reaction depends upon the magnitude of the positive charge on the carbonyl carbon atom. Hence, any substituent that increases the positive charge on the carbonyl carbon must increase its reactivity towards addition reactions. The introduction ...
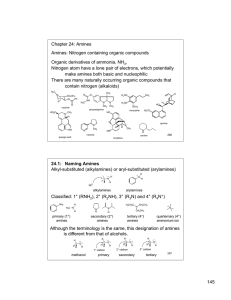
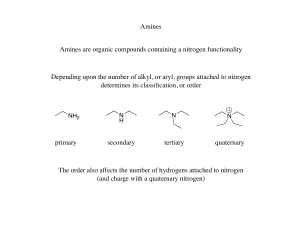
145 Chapter 24: Amines Amines: Nitrogen containing organic
... Table 24.1 (p. 899): pKa values of ammonium ions Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.25) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably less basic than alkyl amines (pKa ~ 5 or less). The nitr ...
... Table 24.1 (p. 899): pKa values of ammonium ions Alkyl ammonium ions, R3NH+ X-, have pKa values in the range of 10-11 (ammonium ion, H4N+ X-, has a pKa ~ 9.25) The ammonium ions of aryl amines and heterocyclic aromatic amines are considerably less basic than alkyl amines (pKa ~ 5 or less). The nitr ...
The progression of chiral anions from concepts to applications in
... are sparked when one or two chemical laboratories uncover a new reagent, catalyst or underlying mode of reactivity. But this simplistic view, which echoes the romantic notion of a ‘Eureka!’-shouting scientist, is clearly not the whole story. In many cases, it may be more illuminating to consider the ...
... are sparked when one or two chemical laboratories uncover a new reagent, catalyst or underlying mode of reactivity. But this simplistic view, which echoes the romantic notion of a ‘Eureka!’-shouting scientist, is clearly not the whole story. In many cases, it may be more illuminating to consider the ...
Q4) How the following conversions can be carried out?
... ANS a. In phenol the electron withdrawing inductive effect of –OH group is opposed by electron releasing the resonance effect of –OH. b. The lone pair on oxygen of –OH in phenol is being shared with benzene ring through resonance. Thus, lone pair is not fully present on oxygen and hence phenols do n ...
... ANS a. In phenol the electron withdrawing inductive effect of –OH group is opposed by electron releasing the resonance effect of –OH. b. The lone pair on oxygen of –OH in phenol is being shared with benzene ring through resonance. Thus, lone pair is not fully present on oxygen and hence phenols do n ...
Metal–ligand multiple bonds as frustrated Lewis - Beilstein
... metathesis at zirconium(IV). As with the Schrock neopentylidene, the reaction proceeds through a four-membered metallacycle, which eliminates the organic product through a [2 + 2] cycloreversion (Scheme 4) [28]. Other early metal imides may demonstrate similar reactivity, as seen in a reaction repor ...
... metathesis at zirconium(IV). As with the Schrock neopentylidene, the reaction proceeds through a four-membered metallacycle, which eliminates the organic product through a [2 + 2] cycloreversion (Scheme 4) [28]. Other early metal imides may demonstrate similar reactivity, as seen in a reaction repor ...
Reaction Mechanism of d-metal complexes Chapter 20
... 1. Identities of Ls effect rates of reaction; incoming L has greatest effect on rate 2. Keq of displacement reaction can rank Ls in order of their strength as Lewis bases 3. For kinetics, concept of nuclephilicity is used (instead of equilibrium concept of basicit 4. Nuclephilicity: rate of attack ...
... 1. Identities of Ls effect rates of reaction; incoming L has greatest effect on rate 2. Keq of displacement reaction can rank Ls in order of their strength as Lewis bases 3. For kinetics, concept of nuclephilicity is used (instead of equilibrium concept of basicit 4. Nuclephilicity: rate of attack ...
Full-Text PDF
... emergent fluorinated substituents, like α-fluorinated ethers, has been observed within recent years [5–8]. While aromatic trifluoromethyl ethers, since their first publication [9] in 1955, have been extensively studied and widely used as pharmaceuticals and crop protection agents, as well as critica ...
... emergent fluorinated substituents, like α-fluorinated ethers, has been observed within recent years [5–8]. While aromatic trifluoromethyl ethers, since their first publication [9] in 1955, have been extensively studied and widely used as pharmaceuticals and crop protection agents, as well as critica ...
Two-electron Quenching of Dinuclear Ruthenium(II)
... cubic crystals were able to obtain from the good purity product for single crystal analysis. The torsion angle between the planes of the subunit phenanthrolines is about 66 degrees. A dinuclear ruthenium (II) polypyridyl complex, (phen)2Ru(diphen)Ru(phen)24+, was synthesized by using polymeric ruthe ...
... cubic crystals were able to obtain from the good purity product for single crystal analysis. The torsion angle between the planes of the subunit phenanthrolines is about 66 degrees. A dinuclear ruthenium (II) polypyridyl complex, (phen)2Ru(diphen)Ru(phen)24+, was synthesized by using polymeric ruthe ...
Proceeding of the National Academy of Sciences of the USA 2006
... ne of the most fascinating catalytic reactions in biology is the reduction of dinitrogen to ammonia by various nitrogenases, the first and most studied being one whose core contains seven irons and one molybdenum (1–4). ‘‘Alternative’’ nitrogenases are now known, one that contains vanadium instead o ...
... ne of the most fascinating catalytic reactions in biology is the reduction of dinitrogen to ammonia by various nitrogenases, the first and most studied being one whose core contains seven irons and one molybdenum (1–4). ‘‘Alternative’’ nitrogenases are now known, one that contains vanadium instead o ...
High-Oxidation-State Palladium Catalysis: New Reactivity for
... and propargyl substituents, among others.[5–7] As a consequence of the carbon-rich coordination sphere of isolated PdIV complexes, C C bond formation is typically their dominant reaction. For the parent complex 2, a detailed study suggested that iodide dissociation preceded ethane formation. Hence, ...
... and propargyl substituents, among others.[5–7] As a consequence of the carbon-rich coordination sphere of isolated PdIV complexes, C C bond formation is typically their dominant reaction. For the parent complex 2, a detailed study suggested that iodide dissociation preceded ethane formation. Hence, ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.