Carbon nanocomposite catalysts for oxygen reduction: from nitrogen
... supports during the thermal decomposition process are the keys to achieving high activity and durability. Figure 2a and b show superior activity and durability to Pt/ C for the best Fe–N–C in alkaline media (0.1 M NaOH). However, in more challenging acid media, NPMCs with Fe– N–C formulations cannot ...
... supports during the thermal decomposition process are the keys to achieving high activity and durability. Figure 2a and b show superior activity and durability to Pt/ C for the best Fe–N–C in alkaline media (0.1 M NaOH). However, in more challenging acid media, NPMCs with Fe– N–C formulations cannot ...
Triruthenium and triosmium carbonyl clusters containing chiral
... the reaction pathway that leads to the mixture of 1 and 2 from [Ru3 (CO)12 ] and levamisolium chloride, we treated the activated cluster [Ru3 (CO)10 (MeCN)2 ] with levamisolium chloride in dichloromethane at room temperature ([Ru3 (CO)12 ] does not react with levamisolium chloride at this temperatur ...
... the reaction pathway that leads to the mixture of 1 and 2 from [Ru3 (CO)12 ] and levamisolium chloride, we treated the activated cluster [Ru3 (CO)10 (MeCN)2 ] with levamisolium chloride in dichloromethane at room temperature ([Ru3 (CO)12 ] does not react with levamisolium chloride at this temperatur ...
- Thieme Connect
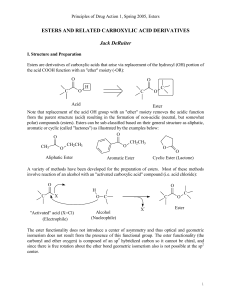
... the conversion of carboxylic acids into their esters using excess triphenylphosphine dibromide, base, and alcohol. The reaction gave the esterified product in moderate to high yields. (F) Preparation of N-Nitrosamines and Azides: The PPh3Br2 in combination with n-Bu4NNO2 has been applied successfull ...
... the conversion of carboxylic acids into their esters using excess triphenylphosphine dibromide, base, and alcohol. The reaction gave the esterified product in moderate to high yields. (F) Preparation of N-Nitrosamines and Azides: The PPh3Br2 in combination with n-Bu4NNO2 has been applied successfull ...
Synthesis of Heterocycles from Anthranilic acid
... common benzodiazepine drugs (such as diazepam, Valium®) which are 1,4benzodiazepine-2-ones. Capture of the dianions with aldehydes or ketones, led to 1,2dihydroquinazolines. Unsubstituted imine anions could be formed by treatment of anthranilonitrile with diisobutylaluminium hydride. Also in this ca ...
... common benzodiazepine drugs (such as diazepam, Valium®) which are 1,4benzodiazepine-2-ones. Capture of the dianions with aldehydes or ketones, led to 1,2dihydroquinazolines. Unsubstituted imine anions could be formed by treatment of anthranilonitrile with diisobutylaluminium hydride. Also in this ca ...
A review of new developments in the Friedel–Crafts - Beilstein
... The Friedel–Crafts benzylation of arenes using benzyl alcohols was discussed in the previous chapter. Even though it renders a convenient and environmental benign approach to 1,1diarylalkanes, there is still one stoichiometric side product formed during this transformation, namely water. Waste water ...
... The Friedel–Crafts benzylation of arenes using benzyl alcohols was discussed in the previous chapter. Even though it renders a convenient and environmental benign approach to 1,1diarylalkanes, there is still one stoichiometric side product formed during this transformation, namely water. Waste water ...
Palladium Complexes Bearing Novel Strongly Bent Trans
... distortion from the square-planar geometry was previously documented, this arrangement is very rare and atypical for the majority of bidentate ligands. For example, similar parameters have been observed for trans-coordinated Xantphos ligands and some palladium complexes bearing various metalloligand ...
... distortion from the square-planar geometry was previously documented, this arrangement is very rare and atypical for the majority of bidentate ligands. For example, similar parameters have been observed for trans-coordinated Xantphos ligands and some palladium complexes bearing various metalloligand ...
Synthesis and Spectroscopic Characterization of
... coordination of transition metals, leaving the 2 axial sites open for ancillary ligands. By incorporating additional groups around the phenol portion of the ligand, such as tert -butyl, the ligands can be made highly soluble in common organic solvents.3 Incorporation of hydrophilic groups may also l ...
... coordination of transition metals, leaving the 2 axial sites open for ancillary ligands. By incorporating additional groups around the phenol portion of the ligand, such as tert -butyl, the ligands can be made highly soluble in common organic solvents.3 Incorporation of hydrophilic groups may also l ...
Oxidative Alihatic Carbon-Carbon Bond Cleavage Reactions
... Rather, ferrous ion-mediated hydration of a vicinal triketone intermediate was the key factor in determining the regioselectivity of the C-C cleavage reaction. We have developed a high-yielding synthetic route to protected precursors of C(1)H acireductones. Preparation of the complexes [(6Ph2TPA)M(P ...
... Rather, ferrous ion-mediated hydration of a vicinal triketone intermediate was the key factor in determining the regioselectivity of the C-C cleavage reaction. We have developed a high-yielding synthetic route to protected precursors of C(1)H acireductones. Preparation of the complexes [(6Ph2TPA)M(P ...
7: Reactions of Haloalkanes, Alcohols, and Amines. Nucleophilic
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
... In fact, when R3 C-Br has fewer than two CH3 groups, it does not react at all by the S N1 mechanism (see Figure7.13). These changes in SN1 rates result from the effect of alkyl groups such as CH3 on the stability of R3 C+ that forms in the first step of the SN1 mechanism. Carbocation Stability. The ...
Organometallic Methods for Forming and Cleaving Carbon
... chloride to di-t-butyl ketone followed by exchange of both the benzyl and the ketone moiety with another substrate. Similar experiments were performed with phenylmagnesium bromide and t-butylmagnesium chloride, but in these two cases the Grignard addition reaction did not show any sign of a reverse ...
... chloride to di-t-butyl ketone followed by exchange of both the benzyl and the ketone moiety with another substrate. Similar experiments were performed with phenylmagnesium bromide and t-butylmagnesium chloride, but in these two cases the Grignard addition reaction did not show any sign of a reverse ...
Reactions of Alcohols
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.