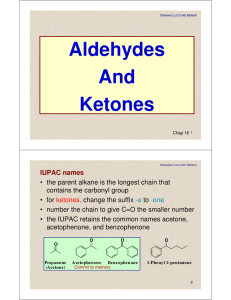
Aldehydes And Ketones
... or other Lewis acid to form a resonancestabilized cation-– protonation increases the electron deficiency of the carbonyl carbon and makes it more reactive toward nucleophiles R R ...
... or other Lewis acid to form a resonancestabilized cation-– protonation increases the electron deficiency of the carbonyl carbon and makes it more reactive toward nucleophiles R R ...
Physical Organic Chemistry
... • the rate of catalysis is given by the turnover number • a reaction may alternatively be “promoted” (accelerated, rather than catalysed) by an additive that is consumed • a heterogeneous catalyst is not dissolved in solution; catalysis typically takes place on its surface • a homogeneous cataly ...
... • the rate of catalysis is given by the turnover number • a reaction may alternatively be “promoted” (accelerated, rather than catalysed) by an additive that is consumed • a heterogeneous catalyst is not dissolved in solution; catalysis typically takes place on its surface • a homogeneous cataly ...
ionic liquids: preparations and limitations
... Ionic liquids are considered as an ideal alternative to volatile organic solvents and chemical industries in the future, because they are non-volatile. Ionic liquids are also considered as new novel chemical agents and widely regarded as a greener alternative to many commonly used solvents. Ionic li ...
... Ionic liquids are considered as an ideal alternative to volatile organic solvents and chemical industries in the future, because they are non-volatile. Ionic liquids are also considered as new novel chemical agents and widely regarded as a greener alternative to many commonly used solvents. Ionic li ...
Nucleophilic ring opening of aziridines
... The aziridine functionality, or alternatively named the azaethylene or ethylenimine unit, represents one of the most valuable three membered ring systems in modern synthetic chemistry, because of its widely recognized versatility as a significant building block for chemical bond elaborations and fun ...
... The aziridine functionality, or alternatively named the azaethylene or ethylenimine unit, represents one of the most valuable three membered ring systems in modern synthetic chemistry, because of its widely recognized versatility as a significant building block for chemical bond elaborations and fun ...
Article - Sociedade Brasileira de Química
... next attempt, we applied this protocol for the reduction of two ketones such as 9-fluorenone or 4-phenyl-2-butanone versus acetophenone; here, it was found that 9-fluorenone and 4-phenyl-2-butanone were reduced in high chemoselectivity (Table 4). Regioselective 1,2-reduction of α,β-unsaturated aldeh ...
... next attempt, we applied this protocol for the reduction of two ketones such as 9-fluorenone or 4-phenyl-2-butanone versus acetophenone; here, it was found that 9-fluorenone and 4-phenyl-2-butanone were reduced in high chemoselectivity (Table 4). Regioselective 1,2-reduction of α,β-unsaturated aldeh ...
Chemistry 360 - Athabasca University
... beginning the labs. In order to successfully complete the laboratory component, please be aware of the following 4 step process of instruction. It is the intention of this Chem360 Report Workbook to provide you with the means of completing all four steps. Step 1: First we tell you what you are going ...
... beginning the labs. In order to successfully complete the laboratory component, please be aware of the following 4 step process of instruction. It is the intention of this Chem360 Report Workbook to provide you with the means of completing all four steps. Step 1: First we tell you what you are going ...
Patterning of Surfaces by Locally Catalyzed Chemical Reactions
... relates to the fact that particles of this size can be used as catalysts for a large variety of chemical reactions. An especially surprising fact is that macroscopically non-catalytic materials can become catalytic active when their size is decreased below 10 nm. Gold, for example, is catalytically ...
... relates to the fact that particles of this size can be used as catalysts for a large variety of chemical reactions. An especially surprising fact is that macroscopically non-catalytic materials can become catalytic active when their size is decreased below 10 nm. Gold, for example, is catalytically ...
isomeria geometrica
... Stereocenters • Any atom at which the exchange of two groups yields a stereoisomer. • Examples: • Asymmetric carbons • Double-bonded carbons in cis-trans isomers ...
... Stereocenters • Any atom at which the exchange of two groups yields a stereoisomer. • Examples: • Asymmetric carbons • Double-bonded carbons in cis-trans isomers ...
PHENOL - Gneet's
... The above reaction is laboratory method for preparation of phenol 2. Hydrolysis of diazonium salt ...
... The above reaction is laboratory method for preparation of phenol 2. Hydrolysis of diazonium salt ...
Intermetal Oxygen, Sulfur, Selenium, and Nitrogen Atom Transfer
... L. Keith Woo was born in Montana and is a graduate of Harvey Mudd College (B.S.) and Stanford University (Ph.D.). Following a postdoctoral research appointment at the University of Wisconsin-Madison, he joined the faculty at Iowa State University In 1986 and Is currently an associate professor and a ...
... L. Keith Woo was born in Montana and is a graduate of Harvey Mudd College (B.S.) and Stanford University (Ph.D.). Following a postdoctoral research appointment at the University of Wisconsin-Madison, he joined the faculty at Iowa State University In 1986 and Is currently an associate professor and a ...
研 究 業 績 リ ス ト
... (92) Ruthenium-Catalyzed Intramolecular Cyclization of 3-Butyne-1,2-diols into Furans Y. Yada, Y. Miyake, and Y. Nishibayashi Org. Lett., to be submitted. (93) Novel Monophosphido-Bridged Diruthenium Complexes: Efficient Preparative Method and Their Catalytic Activity toward Condensation of Aldehyde ...
... (92) Ruthenium-Catalyzed Intramolecular Cyclization of 3-Butyne-1,2-diols into Furans Y. Yada, Y. Miyake, and Y. Nishibayashi Org. Lett., to be submitted. (93) Novel Monophosphido-Bridged Diruthenium Complexes: Efficient Preparative Method and Their Catalytic Activity toward Condensation of Aldehyde ...
Ring-closing metathesis

Ring-closing metathesis, or RCM, is a widely used variation of olefin metathesis in organic chemistry for the synthesis of various unsaturated rings via the intramolecular metathesis of two terminal alkenes, which forms the cycloalkene as the E- or Z- isomers and volatile ethylene.The most commonly synthesized ring sizes are between 5-7 atoms; however, reported syntheses include 45- up to 90- membered macroheterocycles. These reactions are metal-catalyzed and proceed through a metallacyclobutane intermediate. It was first published by Dider Villemin in 1980 describing the synthesis of an Exaltolide precursor, and later become popularized by Robert H. Grubbs and Richard R. Schrock, who shared the Nobel Prize in Chemistry, along with Yves Chauvin, in 2005 for their combined work in olefin metathesis. RCM is a favorite among organic chemists due to its synthetic utility in the formation of rings, which were previously difficult to access efficiently, and broad substrate scope. Since the only major by-product is ethylene, these reactions may also be considered atom economic, an increasingly important concern in the development of green chemistry.There are several reviews published on ring-closing metathesis.