organic chemistry reaction scheme
... *Note: Lithium aluminium hydride (or Lithium tetrahydridoaluminate(III)), LiAlH4, is one of the few reagents that can reduce an acid to an alcohol; the initial product is an alkoxide which the alcohol is liberated by hydrolysis. The –H ion acts as a nucleophile, and can attack the carbon atom of the ...
... *Note: Lithium aluminium hydride (or Lithium tetrahydridoaluminate(III)), LiAlH4, is one of the few reagents that can reduce an acid to an alcohol; the initial product is an alkoxide which the alcohol is liberated by hydrolysis. The –H ion acts as a nucleophile, and can attack the carbon atom of the ...
Chapter 18 Reactions of aromatics
... Mechanism of NBS (Radical) Reaction • Abstraction of a benzylic hydrogen atom generates an intermediate benzylic radical • Reacts with Br2 to yield product • Br· radical cycles back into reaction to carry chain • Br2 produced from reaction of HBr with NBS ...
... Mechanism of NBS (Radical) Reaction • Abstraction of a benzylic hydrogen atom generates an intermediate benzylic radical • Reacts with Br2 to yield product • Br· radical cycles back into reaction to carry chain • Br2 produced from reaction of HBr with NBS ...
Improved Synthesis of (3E,6Z,9Z)-1,3,6,9
... of the two species. Thus, a trapping method that is selective for winter moth would be desirable. A geometric isomer of the pheromone, (3E,6Z,9Z)-1,3,6,9-nonadecatetraene (2), can reportedly inhibit attraction of Bruce spanworm to traps without affecting winter moth catch, but use of the pheromone a ...
... of the two species. Thus, a trapping method that is selective for winter moth would be desirable. A geometric isomer of the pheromone, (3E,6Z,9Z)-1,3,6,9-nonadecatetraene (2), can reportedly inhibit attraction of Bruce spanworm to traps without affecting winter moth catch, but use of the pheromone a ...
Synthesis_of_Organometallic_Compounds
... • Ruthenium hydride complexes are also catalysts for organic reactions such as the coupling reaction of alkenes with terminal alkynes, the [2 + 2] cycloaddition of norbornene with alkynes, Tishchenko-type reactions, and the catalytic insertion of olefins into the ortho C—H bond of ...
... • Ruthenium hydride complexes are also catalysts for organic reactions such as the coupling reaction of alkenes with terminal alkynes, the [2 + 2] cycloaddition of norbornene with alkynes, Tishchenko-type reactions, and the catalytic insertion of olefins into the ortho C—H bond of ...
Chapter 13 - U of L Class Index
... Primary alcohols are oxidized to produce aldehydes and water. Oxidation occurs by removing two atoms of hydrogen, one from the hydroxyl group and a one from the carbon atom that is bonded to the hydroxyl group. The oxidation requires an oxidizing agent such as O2, KMnO4, H2CrO4, or K2Cr2O7. The oxid ...
... Primary alcohols are oxidized to produce aldehydes and water. Oxidation occurs by removing two atoms of hydrogen, one from the hydroxyl group and a one from the carbon atom that is bonded to the hydroxyl group. The oxidation requires an oxidizing agent such as O2, KMnO4, H2CrO4, or K2Cr2O7. The oxid ...
aldehydes and ketones
... • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. • Examples: ...
... • Using the root alkane name, drop the –e ending and change to –one. • Number the longest carbon chain so the C=O group has the lowest number. • Name and number other substituents as before. • Examples: ...
Document
... course, iodosobenzene was quantitively reduced to iodobenzene. A plausible mechanism of this IOB mediated conversion of 1 to 2, outlined in Scheme 2, is analogous to that suggested by Kita et al. for the oxidative conversion of alcohols into esters. Reactive I(III) species 3, which is generated in s ...
... course, iodosobenzene was quantitively reduced to iodobenzene. A plausible mechanism of this IOB mediated conversion of 1 to 2, outlined in Scheme 2, is analogous to that suggested by Kita et al. for the oxidative conversion of alcohols into esters. Reactive I(III) species 3, which is generated in s ...
CHAPTER 17: Carbonyl group (1)
... 17.8 Acetals as protecting groups Due to interference of functional groups during a reaction we often need to transform them to "unreactive species". This is accomplished using "protecting groups" which should be inert to the subsequent reactions. At the end of the synthetic strategy, these protecti ...
... 17.8 Acetals as protecting groups Due to interference of functional groups during a reaction we often need to transform them to "unreactive species". This is accomplished using "protecting groups" which should be inert to the subsequent reactions. At the end of the synthetic strategy, these protecti ...
Introduction to Chemical Reactions
... 2 Mg + O2 → 2 MgO Magnesium atoms and oxygen gas molecules combine to form a single new product Magnesium oxide is the product of the reaction In a moment, we will also see that this reaction can also be classified as a combustion reaction ...
... 2 Mg + O2 → 2 MgO Magnesium atoms and oxygen gas molecules combine to form a single new product Magnesium oxide is the product of the reaction In a moment, we will also see that this reaction can also be classified as a combustion reaction ...
Fundamentals of Organic Chemistry
... name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomers b. Conformational isomers c. Stereoisomers (cis/trans) as applied to cycli ...
... name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomers b. Conformational isomers c. Stereoisomers (cis/trans) as applied to cycli ...
Fundamentals of Organic Chemistry
... name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomers b. Conformational isomers c. Stereoisomers (cis/trans) as applied to cycli ...
... name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomers b. Conformational isomers c. Stereoisomers (cis/trans) as applied to cycli ...
Aldehydes Ketones
... Direct addition (aka 1,2 addition) occurs when a nucleophile attacks the carbon in the carbonyl directly. Conjugate addition (aka 1,4 addition) occurs when the nucleophile attacks the carbonyl indirectly by attacking the second carbon away from the carbonyl group, called the beta carbon, in an u ...
... Direct addition (aka 1,2 addition) occurs when a nucleophile attacks the carbon in the carbonyl directly. Conjugate addition (aka 1,4 addition) occurs when the nucleophile attacks the carbonyl indirectly by attacking the second carbon away from the carbonyl group, called the beta carbon, in an u ...
File - Rasapalli Research Group
... Can be accomplished by inorganic reagents, such as KMnO4, CrO3, and Na2Cr2O7 or by more selective, expensive reagents ...
... Can be accomplished by inorganic reagents, such as KMnO4, CrO3, and Na2Cr2O7 or by more selective, expensive reagents ...
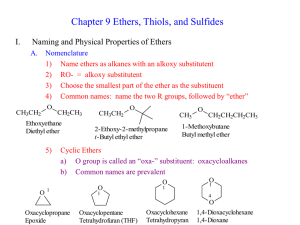
3. Ethers
... •Draw Methoxy butane •Draw Diethoxy benzene •Draw Phenoxy phenol •___________________________________ ether is used as a octane enhancer in petrol. •It is also used in a: • Aldehydes and Ketones: Contain a: –The C=O group is the: ...
... •Draw Methoxy butane •Draw Diethoxy benzene •Draw Phenoxy phenol •___________________________________ ether is used as a octane enhancer in petrol. •It is also used in a: • Aldehydes and Ketones: Contain a: –The C=O group is the: ...
CH_13_5_Disaccharides
... • a disaccharide also known as malt sugar • composed of two D-glucose molecules • obtained from the hydrolysis of starch • used in cereals, candies, and brewing • found in both the and β forms ...
... • a disaccharide also known as malt sugar • composed of two D-glucose molecules • obtained from the hydrolysis of starch • used in cereals, candies, and brewing • found in both the and β forms ...
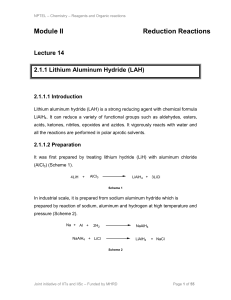
Module II Reduction Reactions
... temperature. The DIBAL-H transfer one hydride to the ester group and forms a tetrahedral intermediate which is stable at low temperature. The hydrolytic work up of the intermediate gives the desired aldehydes (Scheme 13). The presence of alkoxy or amino group to the close proximity can stabilize the ...
... temperature. The DIBAL-H transfer one hydride to the ester group and forms a tetrahedral intermediate which is stable at low temperature. The hydrolytic work up of the intermediate gives the desired aldehydes (Scheme 13). The presence of alkoxy or amino group to the close proximity can stabilize the ...
PDF of this page - Oakland Community College
... is the systematic study of the chemistry of carbon compounds. Topics include structure and properties of carbon containing compounds, nomenclature, acid-base theory, stereochemistry, nucleophilic substitution and elimination reactions, addition reactions involving alkenes and alkynes, radical chemis ...
... is the systematic study of the chemistry of carbon compounds. Topics include structure and properties of carbon containing compounds, nomenclature, acid-base theory, stereochemistry, nucleophilic substitution and elimination reactions, addition reactions involving alkenes and alkynes, radical chemis ...
Thiobenzoate Photochemistry
... transfer could result in the photoreduction of the thiocarbonyl group. Triphenylamine is a tertiary amine without -C-H bonds. It will reveal what happens in the reaction when the radical is denied a facile a H-atom source. Radical coupling may be ultimate result. Finally, trimethylamine offers an ...
... transfer could result in the photoreduction of the thiocarbonyl group. Triphenylamine is a tertiary amine without -C-H bonds. It will reveal what happens in the reaction when the radical is denied a facile a H-atom source. Radical coupling may be ultimate result. Finally, trimethylamine offers an ...
Montmorillonite: An efficient, heterogeneous and
... • May lower the activation energy of a reaction by stabilizing the transition state • May act as a general acid or base • Environmentally benign • Use of clays as catalysts allows for them to be recycled, which further increases their economic efficiency. • Furthermore, reactions that are catalyzed ...
... • May lower the activation energy of a reaction by stabilizing the transition state • May act as a general acid or base • Environmentally benign • Use of clays as catalysts allows for them to be recycled, which further increases their economic efficiency. • Furthermore, reactions that are catalyzed ...
Ch 9 Lecture 2
... 1) Same molecular formula as Alcohol: CnH2n+2O 2) No Hydrogen Bonding is possible in R—O—R 3) Boiling Points are much lower than alcohols, more like haloalkanes 4) Water solubility much less than alcohols a) MeOMe and EtOEt have some water solubility b) Larger ethers are insoluble, very much like al ...
... 1) Same molecular formula as Alcohol: CnH2n+2O 2) No Hydrogen Bonding is possible in R—O—R 3) Boiling Points are much lower than alcohols, more like haloalkanes 4) Water solubility much less than alcohols a) MeOMe and EtOEt have some water solubility b) Larger ethers are insoluble, very much like al ...
Physical Properties OF Aldehydes And Ketones
... know the common and IUPAC nomenclature of aldehydes and ketones Know the physical properties of aldehydes and ketones Know how to synthesize an aldehyde or a ketone from a compound without that functionality. Know the different nucleophilic attack reactions at the carbonyl carbon and the spec ...
... know the common and IUPAC nomenclature of aldehydes and ketones Know the physical properties of aldehydes and ketones Know how to synthesize an aldehyde or a ketone from a compound without that functionality. Know the different nucleophilic attack reactions at the carbonyl carbon and the spec ...
File
... position decreases the electron density in the O−H bond. As a result, it is easier to lose a proton. Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid. On the other hand, methoxy group is an electron-releasing grou ...
... position decreases the electron density in the O−H bond. As a result, it is easier to lose a proton. Also, the o-nitrophenoxide ion formed after the loss of protons is stabilized by resonance. Hence, ortho nitrophenol is a stronger acid. On the other hand, methoxy group is an electron-releasing grou ...
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to the
... Reduction to an aldehyde can be accomplished by using a more reactive carboxylic acid derivatives such as an acyl chloride, ester or nitrile and a less reactive hydride source ...
... Reduction to an aldehyde can be accomplished by using a more reactive carboxylic acid derivatives such as an acyl chloride, ester or nitrile and a less reactive hydride source ...
102 Lecture Ch14a
... can H-bond with themselves and with other alcohols or water - Small alcohols (4 or less C’s) are soluble in water - Phenol is soluble in water (even with 6 C’s) because it partially ionizes in water (it’s a weak acid) - Alcohols and phenols have relatively high boiling points • Thiols are much less ...
... can H-bond with themselves and with other alcohols or water - Small alcohols (4 or less C’s) are soluble in water - Phenol is soluble in water (even with 6 C’s) because it partially ionizes in water (it’s a weak acid) - Alcohols and phenols have relatively high boiling points • Thiols are much less ...
CH_12_3_Reactions_Alcohols_Thiols
... Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition ...
... Chemistry: An Introduction to General, Organic, and Biological Chemistry, Eleventh Edition ...
Elias James Corey

Elias James ""E.J."" Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry ""for his development of the theory and methodology of organic synthesis"", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.