Organic Chemistry Chapter 2 - Snow College | It's SNOWing
... • The greater the number of branches the lower the boiling point • The greater number of electronegative atoms the higher the boiling point ...
... • The greater the number of branches the lower the boiling point • The greater number of electronegative atoms the higher the boiling point ...
Carbonyl Condensation Reactions
... conjugated enone as it is formed, especially if reaction conditions are pushed, eg high temperature. Mixed or "crossed" Aldol Reactions — If two different carbonyl compounds are allowed to react in an aldol reaction four products usually result; each carbonyl compound forms an enolate and each enola ...
... conjugated enone as it is formed, especially if reaction conditions are pushed, eg high temperature. Mixed or "crossed" Aldol Reactions — If two different carbonyl compounds are allowed to react in an aldol reaction four products usually result; each carbonyl compound forms an enolate and each enola ...
Review
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
... that Br2 would. Also unlike Br2, it goes only for the benzylic/allylic position, and doesn’t react significantly with tertiary carbons. Again, if you do this reaction at the allylic position, a mixture of products is possible based on different resonance forms. Reactions with Carbanions Again, a neg ...
Lab 9
... Figure 11. Mechanism of peroxyacid epoxidation. The Experimental Reaction In this experiment, we will prepare an epoxide or oxirane by epoxidizing chlolesterol. Cholesterol is the most common compound in the class of compounds called sterols. As the name implies, sterols contain a hydroxyl (-OH) gro ...
... Figure 11. Mechanism of peroxyacid epoxidation. The Experimental Reaction In this experiment, we will prepare an epoxide or oxirane by epoxidizing chlolesterol. Cholesterol is the most common compound in the class of compounds called sterols. As the name implies, sterols contain a hydroxyl (-OH) gro ...
What is a protecting group?
... functional group in a poly-functional molecule. (b) It should be stable / resistant to the reagents employed in subsequent reaction steps in which the group being masked (protected) is desired to remain deactivated (protected). (c) It should be capable of being selectively removed under mild conditi ...
... functional group in a poly-functional molecule. (b) It should be stable / resistant to the reagents employed in subsequent reaction steps in which the group being masked (protected) is desired to remain deactivated (protected). (c) It should be capable of being selectively removed under mild conditi ...
Alcohols, phenols and ethers
... reactions – Reductions decrease the number of C-O bonds and/or increase the number of C-H bonds in a molecule. ...
... reactions – Reductions decrease the number of C-O bonds and/or increase the number of C-H bonds in a molecule. ...
Hydrocarbon Derivatives:
... Hydrocarbons • Contain only carbon & hydrogen • But carbon can also form strong covalent bonds with other elements such as: O, N, F, Cl, Br, I, S, & P ...
... Hydrocarbons • Contain only carbon & hydrogen • But carbon can also form strong covalent bonds with other elements such as: O, N, F, Cl, Br, I, S, & P ...
DCC-promoted peptide coupling
... amino acids during artificial peptide synthesis. Under standard conditions, it exists in the form of white crystals with a heavy, sweet odor. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and di ...
... amino acids during artificial peptide synthesis. Under standard conditions, it exists in the form of white crystals with a heavy, sweet odor. The low melting point of this material allows it to be melted for easy handling. It is highly soluble in dichloromethane, tetrahydrofuran, acetonitrile and di ...
Advanced Chemistry Grade 12A Sem 2 Overview
... Know that proteins are formed from combinations of 20 different amino acids through peptide bonds and that they have a variety of functions in living things. Know that they can be broken down by hydrolysis into their constituent amino acids, which can be separated by electrophoresis and ionexchange ...
... Know that proteins are formed from combinations of 20 different amino acids through peptide bonds and that they have a variety of functions in living things. Know that they can be broken down by hydrolysis into their constituent amino acids, which can be separated by electrophoresis and ionexchange ...
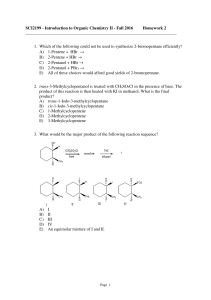
SCI2199 - Introduction to Organic Chemistry II
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
... 7. Which of the following could be used to synthesize 2-bromobutane? A) CH3CH2CH=CH2 + Br2(aq) → B) CH3CH2CHOHCH3 + HBr → C) CH3CH2C≡CH + HBr → D) CH3CH2C≡CH + Br2 → E) More than one of these choices. 8. Which of the alcohols listed below would you expect to react most rapidly with ...
Stereoselective Construction of a β
... (Z)-Cyclic furfuryl ether (Z)-8 was also prepared from aldehyde 3 as shown in Scheme 3. Methacrylate (Z)-4 was obtained by reaction of the anion of ethyl 2-(diphenylphosphono)propionate with 3 using Ando’s protocol.11 In this Horner-Wadsworth-Emmons reaction, (Z)methacrylate was formed as a predomin ...
... (Z)-Cyclic furfuryl ether (Z)-8 was also prepared from aldehyde 3 as shown in Scheme 3. Methacrylate (Z)-4 was obtained by reaction of the anion of ethyl 2-(diphenylphosphono)propionate with 3 using Ando’s protocol.11 In this Horner-Wadsworth-Emmons reaction, (Z)methacrylate was formed as a predomin ...
Alcohols and phenols - Home - KSU Faculty Member websites
... Know the acidic properties of alcohols and phenols know the different methods that can be used to prepare alcohols and phenols. Know the chemical reactions of these compounds ( some reactions are review, others are extensions of the chemistry that will be discussed on chapters 8, 9 & 10 145 Chem ...
... Know the acidic properties of alcohols and phenols know the different methods that can be used to prepare alcohols and phenols. Know the chemical reactions of these compounds ( some reactions are review, others are extensions of the chemistry that will be discussed on chapters 8, 9 & 10 145 Chem ...
Fundamentals of Organic Chemistry
... name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomers b. Conformational isomers c. Stereoisomers (cis/trans) as applied to cycli ...
... name each type of hydrocarbon by means of the IUPAC system. describe the physical properties of hydrocarbons and how these relate to biochemical properties. Use alkanes as model compounds to describe a. Constitutional isomers b. Conformational isomers c. Stereoisomers (cis/trans) as applied to cycli ...
Chapter 8 Alkenes and Alkynes II
... t Reaction of an alkene with hot KMnO4 results in cleavage of the ...
... t Reaction of an alkene with hot KMnO4 results in cleavage of the ...
Alcohols
... The reactivity of NaBH4 is much lower than a free hydride ion, and NaBH4 can be used in protic solvents such as ethanol. The mechanism of a sodium borohydride reduction involves: • Donation of H- to the carbonyl carbon • Simultaneous protonation of the carbonyl oxygen by a solvent molecule • Combin ...
... The reactivity of NaBH4 is much lower than a free hydride ion, and NaBH4 can be used in protic solvents such as ethanol. The mechanism of a sodium borohydride reduction involves: • Donation of H- to the carbonyl carbon • Simultaneous protonation of the carbonyl oxygen by a solvent molecule • Combin ...
Main Menu - MsReenChemistry
... types of racing. The reason for this is that methanol is made of a single chemical. Gasoline, on the other hand, contains many different chemicals, and can vary greatly from one batch to another. Methanol is safer in case of accidental fire than gasoline, because it burns cooler. ...
... types of racing. The reason for this is that methanol is made of a single chemical. Gasoline, on the other hand, contains many different chemicals, and can vary greatly from one batch to another. Methanol is safer in case of accidental fire than gasoline, because it burns cooler. ...
Lewis base-assisted Lewis acid-catalyzed selective
... Acid‐catalyzed dehydration of alcohols has been widely employed for the synthesis of alkenes. However, activated alcohols when employed as substrates in dehydration reactions are often plagued by the lack of alkene selectivity. In this work, the reaction system can be signif ...
... Acid‐catalyzed dehydration of alcohols has been widely employed for the synthesis of alkenes. However, activated alcohols when employed as substrates in dehydration reactions are often plagued by the lack of alkene selectivity. In this work, the reaction system can be signif ...
lecture 3 - aldehydes and ketones
... bond are called electrophiles (literally, "lovers of electrons"). Electrophiles include ions (such as H+ and Fe3+) and neutral molecules (such as AlCl3 and BF3) that are Lewis acids, or electron-pair acceptors. ...
... bond are called electrophiles (literally, "lovers of electrons"). Electrophiles include ions (such as H+ and Fe3+) and neutral molecules (such as AlCl3 and BF3) that are Lewis acids, or electron-pair acceptors. ...
3.8 Aldehydes and ketones
... Oxygen is more electronegative than carbon meaning that the p electrons will be highly distorted towards the oxygen atom as shown above. ...
... Oxygen is more electronegative than carbon meaning that the p electrons will be highly distorted towards the oxygen atom as shown above. ...
O-isopropylideneadenosine (1)
... To a –20°C solution of freshly distilled furan (3.5 g, 52 mmol) in anhydrous THF (20 mL), under argon atmosphere, was added n-BuLi (22 mL, 55 mmol, 2.5 M solution in hexane). The reaction temperature was allowed to warm to room temperature and stirring was mantained for 2 h. Then, the temperature wa ...
... To a –20°C solution of freshly distilled furan (3.5 g, 52 mmol) in anhydrous THF (20 mL), under argon atmosphere, was added n-BuLi (22 mL, 55 mmol, 2.5 M solution in hexane). The reaction temperature was allowed to warm to room temperature and stirring was mantained for 2 h. Then, the temperature wa ...
Chapter 3 Properties of organic compounds
... -ÕLÃÌÌÕÌÊqÊof the OH− using conc HCl (with ZnCl2) or SOCl2 to form a chloroalkane. Substitution is faster for tertiary alcohols than for secondary, and slowest for primary alcohols. Rate of substitution of alcohols is increased by heating the reaction mixture under reflux – this way the reaction ...
... -ÕLÃÌÌÕÌÊqÊof the OH− using conc HCl (with ZnCl2) or SOCl2 to form a chloroalkane. Substitution is faster for tertiary alcohols than for secondary, and slowest for primary alcohols. Rate of substitution of alcohols is increased by heating the reaction mixture under reflux – this way the reaction ...
Chapter 16 Ethers, Epoxides, and Sulfides
... most stable conformation of diethyl ether resembles pentane ...
... most stable conformation of diethyl ether resembles pentane ...
Elias James Corey

Elias James ""E.J."" Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry ""for his development of the theory and methodology of organic synthesis"", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.