Chloroperbenzoic_aci..
... epoxidation) is observed when the bulkier O-t-Bu is located on the allylic carbon (eq 4).18 Due to steric and other factors, the norbornene (11) undergoes selective (99%) epoxidation from the exo face.19 In 7,7-dimethylnorbornene (12), approach to the exo face is effectively blocked by the methyl su ...
... epoxidation) is observed when the bulkier O-t-Bu is located on the allylic carbon (eq 4).18 Due to steric and other factors, the norbornene (11) undergoes selective (99%) epoxidation from the exo face.19 In 7,7-dimethylnorbornene (12), approach to the exo face is effectively blocked by the methyl su ...
103. Oxalates as Activating Groups for Alcohols in Visible Light Photoredox Catalysis: Formation of Quaternary Centers by Redox-Neutral Fragment Coupling
... Activation of the tertiary alcohol of known intermediate 6917 is particularly challenging because the trans-decalin ring system places the tertiary alcohol in a 1,3-diaxial relationship with the angular methyl substituent. This severe steric interaction had previously prevented the preparation of th ...
... Activation of the tertiary alcohol of known intermediate 6917 is particularly challenging because the trans-decalin ring system places the tertiary alcohol in a 1,3-diaxial relationship with the angular methyl substituent. This severe steric interaction had previously prevented the preparation of th ...
full size
... aldehydes are less volatile (higher boiling) than alkanes or ethers but are more volatile than alcohols or carboxylic acids. They are slightly less soluble in water than the alcohols of similar molecular weight. δ− ¾Simple aldehydes have very O pungent and irritating odors and δ+ C are toxic. Aldehy ...
... aldehydes are less volatile (higher boiling) than alkanes or ethers but are more volatile than alcohols or carboxylic acids. They are slightly less soluble in water than the alcohols of similar molecular weight. δ− ¾Simple aldehydes have very O pungent and irritating odors and δ+ C are toxic. Aldehy ...
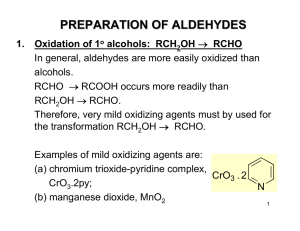
PREPARATION OF ALDEHYDES
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
... EXAMPLES OF NUCLEOPHILIC ADDITION TO ALDEHYDES & KETONES Addition of HCN (neutral-basic conditions). CN Ө is a very good nucleophile (ionic nucleophile). The use of the actual compound HCN is not experimentally feasible, as it is a lethal gas, bp 26 oC. Addition of the elements of HCN to a C=O grou ...
Chem 314 Preorganic Evaluation
... 1oRX will produce mainly SN2 product excet for mostly E2 with the sterically hindered and highly basic potassium t-butoxide and generally more E2 occurs from the less hindered side of the RX allylic & benzylic RX are very reactive if a conjugated pi bond can form complete substitution at Cα (3o RX) ...
... 1oRX will produce mainly SN2 product excet for mostly E2 with the sterically hindered and highly basic potassium t-butoxide and generally more E2 occurs from the less hindered side of the RX allylic & benzylic RX are very reactive if a conjugated pi bond can form complete substitution at Cα (3o RX) ...
Hydroxyl Compounds
... i) alcohols with short carbon chains (such as methanol, ethanol, and propanol) dissolve in water. - when alcohols dissolve in water, hydrogen bonds are formed between the –OH group of the alcohol molecule and the –OH group of the water molecule. ii) the solubility of alcohols in water decreases shar ...
... i) alcohols with short carbon chains (such as methanol, ethanol, and propanol) dissolve in water. - when alcohols dissolve in water, hydrogen bonds are formed between the –OH group of the alcohol molecule and the –OH group of the water molecule. ii) the solubility of alcohols in water decreases shar ...
Mock Exam One
... a.) LiAlH4 and a Ketone b.) CH3CH2MgBr and an Aldehyde c.) 2-butene and Hg(OAc)2, H2O followed by NaBH4 d.) All of these. 3.) Which of the following terms best describes the reactive nature of the Grignard ...
... a.) LiAlH4 and a Ketone b.) CH3CH2MgBr and an Aldehyde c.) 2-butene and Hg(OAc)2, H2O followed by NaBH4 d.) All of these. 3.) Which of the following terms best describes the reactive nature of the Grignard ...
chapter 6-hydroxyl compounds
... i) alcohols with short carbon chains (such as methanol, ethanol, and propanol) dissolve in water. - when alcohols dissolve in water, hydrogen bonds are formed between the –OH group of the alcohol molecule and the –OH group of the water molecule. ii) the solubility of alcohols in water decreases shar ...
... i) alcohols with short carbon chains (such as methanol, ethanol, and propanol) dissolve in water. - when alcohols dissolve in water, hydrogen bonds are formed between the –OH group of the alcohol molecule and the –OH group of the water molecule. ii) the solubility of alcohols in water decreases shar ...
Carbohydrates: Occurrence, Structures and Chemistry
... was purely arbitrary, yet proved to be a fortunate one, since much later, in 1951, it was proven by special X-ray structural analysis [13] that he had made the right choice. The D-aldose family tree is shown in Figure 3, comprising five of the most important ...
... was purely arbitrary, yet proved to be a fortunate one, since much later, in 1951, it was proven by special X-ray structural analysis [13] that he had made the right choice. The D-aldose family tree is shown in Figure 3, comprising five of the most important ...
5 Organic Chemistry
... There are now many polymers derived from plant sources. Using your knowledge of reactions in AS chemistry, suggest a route to poly(ethane) that is derived from a plant source. Include reagents and conditions where appropriate. (6 marks) ...
... There are now many polymers derived from plant sources. Using your knowledge of reactions in AS chemistry, suggest a route to poly(ethane) that is derived from a plant source. Include reagents and conditions where appropriate. (6 marks) ...
CHEMICAL REACTIVITY AND MECHANISMS, AND SUBSTITUTION REACTIONS 1.
... steric effect; larger groups interfere with the approaching nucleophile). SN1 reactions are faster for 3° substrates (because the more stable the carbocation, the faster the reaction; this means 3° > 2° >> 1° > CH3). Vinylic (R2C=CR-) and aromatic substrates are unreactive in either reaction type. A ...
... steric effect; larger groups interfere with the approaching nucleophile). SN1 reactions are faster for 3° substrates (because the more stable the carbocation, the faster the reaction; this means 3° > 2° >> 1° > CH3). Vinylic (R2C=CR-) and aromatic substrates are unreactive in either reaction type. A ...
View/Open
... product on hydrogenation. The results of such an experiment involving platinumcatalyzed hydrogenation of three butene isomers are shown in Fig. 7.2. All three isomers yield the same product—butane—but the heat of reaction is different in each case. On conversion to butane, 1-butene liberates the mos ...
... product on hydrogenation. The results of such an experiment involving platinumcatalyzed hydrogenation of three butene isomers are shown in Fig. 7.2. All three isomers yield the same product—butane—but the heat of reaction is different in each case. On conversion to butane, 1-butene liberates the mos ...
Synthesis of Novel Steroid-Peptoid Hybrid Macrocycles by
... As depicted in Scheme 4 (top), an unidirectional Ugi-MiB approach can be implemented easily by utilizing the lithocholic acid derivative 17 functionalized with an amino group at C-3. This compound was readily prepared from methyl lithocholate according to reported procedures [13,24]. The 3α-OH is re ...
... As depicted in Scheme 4 (top), an unidirectional Ugi-MiB approach can be implemented easily by utilizing the lithocholic acid derivative 17 functionalized with an amino group at C-3. This compound was readily prepared from methyl lithocholate according to reported procedures [13,24]. The 3α-OH is re ...
here
... You get from CH3Br to C2H3N (CH3CN) with –CN. Add 2H2 to CH3CN to get to C2H7N (CH3CH2NH2). React CH3CH2NH2 with CH3Br to get the final product of ...
... You get from CH3Br to C2H3N (CH3CN) with –CN. Add 2H2 to CH3CN to get to C2H7N (CH3CH2NH2). React CH3CH2NH2 with CH3Br to get the final product of ...
Chapter 19. Aldehydes and Ketones
... C=O Proton is lost from N and adds to O to yield a neutral amino alcohol (carbinolamine) Protonation of OH converts into water as the leaving group Result is iminium ion, which loses proton Acid is required for loss of OH – too much acid blocks RNH2 ...
... C=O Proton is lost from N and adds to O to yield a neutral amino alcohol (carbinolamine) Protonation of OH converts into water as the leaving group Result is iminium ion, which loses proton Acid is required for loss of OH – too much acid blocks RNH2 ...
Developments in Synthetic Application of Selenium(IV) Oxide and
... Selenium(IV) oxide allylic hydroxylations are highly regiospecific and occur at the α-position to the more substituted carbon of the double bond with a reactivity order CH2 > CH3 > CH. When the double bond is inside a ring, oxidation occurs in the ring when possible, and in the α-position to the mor ...
... Selenium(IV) oxide allylic hydroxylations are highly regiospecific and occur at the α-position to the more substituted carbon of the double bond with a reactivity order CH2 > CH3 > CH. When the double bond is inside a ring, oxidation occurs in the ring when possible, and in the α-position to the mor ...
View/Open - Minerva Access
... Metal-catalyzed decarboxylative coupling reactions of esters offer new opportunities for formation of C-C bonds with CO2 as the only coproduct. Here I provide an overview of: key solution phase literature; thermochemical considerations for decarboxylation of esters and thermolysis of esters in the a ...
... Metal-catalyzed decarboxylative coupling reactions of esters offer new opportunities for formation of C-C bonds with CO2 as the only coproduct. Here I provide an overview of: key solution phase literature; thermochemical considerations for decarboxylation of esters and thermolysis of esters in the a ...
aldehydes and ketones
... (b) Position isomerism: aliphatic aldehydes do not show position isomerism, because –CHO group is always present at the end of carbon chain. Aromatic aldehyde show position isomerism. Example (c) Metamerism: Higher ketones show metamerism due to presence of different alkyl groups attached to the sam ...
... (b) Position isomerism: aliphatic aldehydes do not show position isomerism, because –CHO group is always present at the end of carbon chain. Aromatic aldehyde show position isomerism. Example (c) Metamerism: Higher ketones show metamerism due to presence of different alkyl groups attached to the sam ...
Chapter 27. Biomolecules: Lipids
... a trans manner In cis-decalin, both groups at the ring-junction positions are on the same side of the two rings In trans-decalin, the groups at the ring junctions are on opposite sides ...
... a trans manner In cis-decalin, both groups at the ring-junction positions are on the same side of the two rings In trans-decalin, the groups at the ring junctions are on opposite sides ...
Chapter 27. Biomolecules: Lipids
... a trans manner In cis-decalin, both groups at the ring-junction positions are on the same side of the two rings In trans-decalin, the groups at the ring junctions are on opposite sides ...
... a trans manner In cis-decalin, both groups at the ring-junction positions are on the same side of the two rings In trans-decalin, the groups at the ring junctions are on opposite sides ...
07. Aldehydes and ketones
... As noted earlier, an aldehyde is partially converted to its enolate anion by bases such as hydroxide ion and alkoxide ions. This type of condensations is character for aldehydes which have hydrogen atoms at the α-carbon atom. ...
... As noted earlier, an aldehyde is partially converted to its enolate anion by bases such as hydroxide ion and alkoxide ions. This type of condensations is character for aldehydes which have hydrogen atoms at the α-carbon atom. ...
Aldehydes and ketones
... In these compounds, the carbonyl group is bound to a nitrogen (an amino group), in addition to either a H-atom or a carbon group (alkyl, cycloalkyl, aromatic). The R’ and R” groups of the amino group may either be H or carbon groups: General formula for an amide: ...
... In these compounds, the carbonyl group is bound to a nitrogen (an amino group), in addition to either a H-atom or a carbon group (alkyl, cycloalkyl, aromatic). The R’ and R” groups of the amino group may either be H or carbon groups: General formula for an amide: ...
- Sacramento - California State University
... Vanadium has been used as a catalyst for polymerization1, oxidation of alcohols2, sulfides3, and more importantly, allylic alcohols. Epoxides are useful building blocks for natural product synthesis and medicinal chemistry because new functional groups can easily be introduced by nucleophilic additi ...
... Vanadium has been used as a catalyst for polymerization1, oxidation of alcohols2, sulfides3, and more importantly, allylic alcohols. Epoxides are useful building blocks for natural product synthesis and medicinal chemistry because new functional groups can easily be introduced by nucleophilic additi ...
Elias James Corey

Elias James ""E.J."" Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry ""for his development of the theory and methodology of organic synthesis"", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.