CBSE Class 12 Chemistry notes and questions for Alcohols Phenols
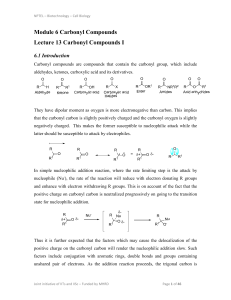
... gives carboxylic acid B (C4H8O2). Compound A when dehydrated with conc. H2SO4 at 443 K gives compound C. Treatment of C with aqueous H2SO4 gives compound D (C4H10O) which is an isomer of A. Compound D is resistant to oxidation but compound A can be easily oxidised. Identify A, B, C and D and write t ...
... gives carboxylic acid B (C4H8O2). Compound A when dehydrated with conc. H2SO4 at 443 K gives compound C. Treatment of C with aqueous H2SO4 gives compound D (C4H10O) which is an isomer of A. Compound D is resistant to oxidation but compound A can be easily oxidised. Identify A, B, C and D and write t ...
research reviews Discovering new arene-catalyzed lithiations
... the addition of an organolithium reagent to an olefin, yielding a new organolithium intermediate having at least two more carbon atoms [50]. A significant advantage of this process is that the new organolithium can then react with an electrophile, such that in only one synthetic operation profound c ...
... the addition of an organolithium reagent to an olefin, yielding a new organolithium intermediate having at least two more carbon atoms [50]. A significant advantage of this process is that the new organolithium can then react with an electrophile, such that in only one synthetic operation profound c ...
kinetic and thermodynamic studies of the oxidation of perfumery
... *E-mail: [email protected] ABSTRACT Oxidation is one of the most important industrial reactions as it yields useful products. Literature survey indicates the use of a variety of organic oxidants for the oxidation of alcohols to the corresponding carbonyl compounds but inorganic oxidants have rare ...
... *E-mail: [email protected] ABSTRACT Oxidation is one of the most important industrial reactions as it yields useful products. Literature survey indicates the use of a variety of organic oxidants for the oxidation of alcohols to the corresponding carbonyl compounds but inorganic oxidants have rare ...
Enol esters: Versatile substrates in synthesis of fine and specialty
... acidic conditions with mostly retention of configuration. Both α-acyloxy ketones and their αhydroxy ketone derivatives, are essential in synthesis of alkaloids, sugars, antibiotics, terpenes and pheromones, for they function as stereodirective groups or chiral synthons. Zhu et al. practised the rear ...
... acidic conditions with mostly retention of configuration. Both α-acyloxy ketones and their αhydroxy ketone derivatives, are essential in synthesis of alkaloids, sugars, antibiotics, terpenes and pheromones, for they function as stereodirective groups or chiral synthons. Zhu et al. practised the rear ...
Chapter 16
... The alkoxide will reform a carbonyl (strong bond) with the good leaving group present ...
... The alkoxide will reform a carbonyl (strong bond) with the good leaving group present ...
Fluorination Chemistry - Sigma
... ©2013 Sigma-Aldrich Co. LLC. All rights reserved. SAFC, SIGMA-ALDRICH, and ALDRICH are trademarks of Sigma-Aldrich Co. LLC, registered in the US and other countries. Add Aldrich is a trademark of Sigma-Aldrich Co. LLC. Celatom is a registered trademark of EP Minerals, LLC. IKA is a registered tradem ...
... ©2013 Sigma-Aldrich Co. LLC. All rights reserved. SAFC, SIGMA-ALDRICH, and ALDRICH are trademarks of Sigma-Aldrich Co. LLC, registered in the US and other countries. Add Aldrich is a trademark of Sigma-Aldrich Co. LLC. Celatom is a registered trademark of EP Minerals, LLC. IKA is a registered tradem ...
Nucleophilic Substitution on the Carbonyl Group
... transfer reaction works well with an acyl halide because the halide ion is a weak base. Thus, an acyl halide has a very good leaving group. Conversely, acyl transfer reactions do not occur with aldehydes and ketones because the leaving group, either a hydride or a carbanion, is generally too strong ...
... transfer reaction works well with an acyl halide because the halide ion is a weak base. Thus, an acyl halide has a very good leaving group. Conversely, acyl transfer reactions do not occur with aldehydes and ketones because the leaving group, either a hydride or a carbanion, is generally too strong ...
Titania-catalysed oxidative dehydrogenation of ethyl lactate
... absence any strong base or solvent. But this is much easier said than done. Addressing this challenge, we screened various simple oxides with the aim of finding a catalyst that can work in the oxidative dehydrogenation of lactic acid. After several experiments, we chose titania (TiO2) as a catalyst. ...
... absence any strong base or solvent. But this is much easier said than done. Addressing this challenge, we screened various simple oxides with the aim of finding a catalyst that can work in the oxidative dehydrogenation of lactic acid. After several experiments, we chose titania (TiO2) as a catalyst. ...
Handout V
... Amines, like ammonia, are strong enough bases that they are completely protonated in dilute acid solutions. Basicity can be increased by increasing the availability of lone pair and stabilizing the resultant positive charge. When one hydrogen of ammonia replaced by methyl group, it stabilizes the re ...
... Amines, like ammonia, are strong enough bases that they are completely protonated in dilute acid solutions. Basicity can be increased by increasing the availability of lone pair and stabilizing the resultant positive charge. When one hydrogen of ammonia replaced by methyl group, it stabilizes the re ...
Chapter 19. Aldehydes and Ketones
... The sequence converts C=O to C=C A phosphorus ylide adds to an aldehyde or ketone to yield a dipolar intermediate called a betaine The intermediate spontaneously decomposes through a four-membered ring to yield alkene and triphenylphosphine oxide, (Ph)3P=O Formation of the ylide is shown below ...
... The sequence converts C=O to C=C A phosphorus ylide adds to an aldehyde or ketone to yield a dipolar intermediate called a betaine The intermediate spontaneously decomposes through a four-membered ring to yield alkene and triphenylphosphine oxide, (Ph)3P=O Formation of the ylide is shown below ...
Free Radical Chemistry and the Preparation of Alkyl
... Oxidations decrease e- density on C (formation of C-O, C-N or C-X bonds) so reactions that form alkyl halides (R-H to R-X) are oxidations ...
... Oxidations decrease e- density on C (formation of C-O, C-N or C-X bonds) so reactions that form alkyl halides (R-H to R-X) are oxidations ...
Alcohols, Phenols , Phenols and Ethers Alcohols
... According to IUPAC system (Unit 12, Class XI), the name of an alcohol is derived from the name of the alkane from which the alcohol is derived, by substituting ‘e’ of alkane with the suffix ‘ol’. The position of substituents are indicated by numerals. For this, the longest carbon chain (parent chai ...
... According to IUPAC system (Unit 12, Class XI), the name of an alcohol is derived from the name of the alkane from which the alcohol is derived, by substituting ‘e’ of alkane with the suffix ‘ol’. The position of substituents are indicated by numerals. For this, the longest carbon chain (parent chai ...
Pincer Complexes. Applications in Catalysis
... of these complexes have shown activity in the dehydrogenation of alkanes to alkenes. However, the extremely low reaction rates and the low turnover numbers or the instability of the employed catalysts under the reaction conditions16 has limited the use of these species. In 1976 Moulton and Shaw repo ...
... of these complexes have shown activity in the dehydrogenation of alkanes to alkenes. However, the extremely low reaction rates and the low turnover numbers or the instability of the employed catalysts under the reaction conditions16 has limited the use of these species. In 1976 Moulton and Shaw repo ...
Organic Compounds containing Oxygen
... alkene too easily. This method is not useful for the praparation of unsymmetrical ethers from primary alcohols because the reaction leads to a mixture of products. ...
... alkene too easily. This method is not useful for the praparation of unsymmetrical ethers from primary alcohols because the reaction leads to a mixture of products. ...
SYNTHESIS OF NEW DICLOFENAC DERIVATIVES BY COUPLING WITH CHALCONE
... both benzene rings in such a way that this system has relatively low redox potentials and has a greater probability of undergoing electron transfer reactions[11]. In addition the colors of these compounds is attributed to the presence of such chromophore (-COCH=CH-) and other auxochromes (Scheme 1). ...
... both benzene rings in such a way that this system has relatively low redox potentials and has a greater probability of undergoing electron transfer reactions[11]. In addition the colors of these compounds is attributed to the presence of such chromophore (-COCH=CH-) and other auxochromes (Scheme 1). ...
Chemistry of DOW Glycol Ether Products
... Glycol Ether Acetates DOWANOL PMA propylene glycol methyl ether acetate is the reaction product of DOWANOL PM with acetic acid or anhydride. In this case, the “PM” designates propylene glycol methyl ether and the “A” designates the acetate resulting from esterification with acetic acid or anhydride. ...
... Glycol Ether Acetates DOWANOL PMA propylene glycol methyl ether acetate is the reaction product of DOWANOL PM with acetic acid or anhydride. In this case, the “PM” designates propylene glycol methyl ether and the “A” designates the acetate resulting from esterification with acetic acid or anhydride. ...
101. Alcohols as alkylating agents in heteroarene C H functionalization
... deoxygenate ribonucleotides, an example of ‘spin-centre shift’2, during which an alcohol C–O bond is cleaved, resulting in a carbon-centred radical intermediate. Although spin-centre shift is a well-understood biochemical process, it is underused by the synthetic organic chemistry community. We wond ...
... deoxygenate ribonucleotides, an example of ‘spin-centre shift’2, during which an alcohol C–O bond is cleaved, resulting in a carbon-centred radical intermediate. Although spin-centre shift is a well-understood biochemical process, it is underused by the synthetic organic chemistry community. We wond ...
Alcohols - La Salle University
... • Ethanol has a caloric value of 7.1Cal/g (fat has a value of 9 Cal/g). • Alcohol can cause a degenerative muscle disease called alcoholic myopathy (3 times more common than cirrhosis). ...
... • Ethanol has a caloric value of 7.1Cal/g (fat has a value of 9 Cal/g). • Alcohol can cause a degenerative muscle disease called alcoholic myopathy (3 times more common than cirrhosis). ...
Alkyl halide
... into alkyl halides • Treat the alcohol with HCl, HBr, or HI • Simplest method • The reaction works best with tertiary alcohols, R3COH • Primary and secondary alcohols react slowly and at higher reaction temperatures ...
... into alkyl halides • Treat the alcohol with HCl, HBr, or HI • Simplest method • The reaction works best with tertiary alcohols, R3COH • Primary and secondary alcohols react slowly and at higher reaction temperatures ...
ch13[1].
... water, adding NaOH to precipitate Ag2O and then adding aqueous ammonia to redissolve silver ion as the silverammonia complex ion. Tollens’ reagent is specific for the oxidation of aldehydes. If done properly, silver deposits on the walls of the container as a silver mirror. O R-C-H + 2 Ag( NH3 ) 2 + ...
... water, adding NaOH to precipitate Ag2O and then adding aqueous ammonia to redissolve silver ion as the silverammonia complex ion. Tollens’ reagent is specific for the oxidation of aldehydes. If done properly, silver deposits on the walls of the container as a silver mirror. O R-C-H + 2 Ag( NH3 ) 2 + ...
Handout VI
... Lithium aluminium hydride is a very powerful reducing agent and easily reduces both aldehydes and ketones to their corresponding alcohols. The reaction involves irreversible hydride transfer to the carbonyl compound to form an aluminium complex (1) which then further reacts with another three molecu ...
... Lithium aluminium hydride is a very powerful reducing agent and easily reduces both aldehydes and ketones to their corresponding alcohols. The reaction involves irreversible hydride transfer to the carbonyl compound to form an aluminium complex (1) which then further reacts with another three molecu ...
Aldehydes And Ketones
... Grignard Reagents – addition of a Grignard reagent to formaldehyde followed by H3O+ gives a 1° ...
... Grignard Reagents – addition of a Grignard reagent to formaldehyde followed by H3O+ gives a 1° ...
Aldehydes and Ketones
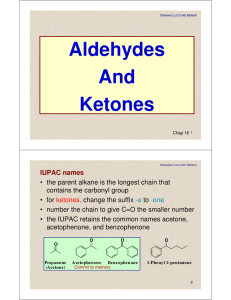
... Naming Aldehydes IUPAC Replace the -e in the alkane name with –al Common Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O ...
... Naming Aldehydes IUPAC Replace the -e in the alkane name with –al Common Add aldehyde to the prefixes form (1C), acet (2C), propion(3), and butry(4C) O ...
Elias James Corey

Elias James ""E.J."" Corey (born July 12, 1928) is an American organic chemist. In 1990, he won the Nobel Prize in Chemistry ""for his development of the theory and methodology of organic synthesis"", specifically retrosynthetic analysis. Regarded by many as one of the greatest living chemists, he has developed numerous synthetic reagents, methodologies and total syntheses and has advanced the science of organic synthesis considerably.




















![ch13[1].](http://s1.studyres.com/store/data/008194698_1-d188c504eac7b7806e762a2340484910-300x300.png)