Methanol Synthesis
... node and rerun the parameter estimation. This is done in file Methanol_MassAction-2.rex. The solution has the same weighted LSQ of 0.14228 obtained before, thus confirming that the removal of that direction does not affect the model predictions. ...
... node and rerun the parameter estimation. This is done in file Methanol_MassAction-2.rex. The solution has the same weighted LSQ of 0.14228 obtained before, thus confirming that the removal of that direction does not affect the model predictions. ...
Lesson 3 Mechanisms of Organic Reactions
... and Br+), carbocations, or neutral molecules such as sulfur trioxide, SO3, or compounds of the general formula R-X, where X is an electron-withdrawing group. Nucleophiles are often, though not always, negatively charged. The most widely known nucleophiles are a hydroxide ion, alkoxide ions (RO-), th ...
... and Br+), carbocations, or neutral molecules such as sulfur trioxide, SO3, or compounds of the general formula R-X, where X is an electron-withdrawing group. Nucleophiles are often, though not always, negatively charged. The most widely known nucleophiles are a hydroxide ion, alkoxide ions (RO-), th ...
Problem Set 12-2: Organic Chemistry
... a. Ethane IMF: dispersion b. Ethanol IMF: hydrogen bonding c. Ethenone IMF: dipole-dipole d. Ethanoic acid IMF: hydrogen bonding e. 1- Chloroethane IMF: mostly dispersion, small amount of dipole-dipole Dispersion forces are the weakest IMF, dipole-dipole are stronger but not as strong as Hydrogen bo ...
... a. Ethane IMF: dispersion b. Ethanol IMF: hydrogen bonding c. Ethenone IMF: dipole-dipole d. Ethanoic acid IMF: hydrogen bonding e. 1- Chloroethane IMF: mostly dispersion, small amount of dipole-dipole Dispersion forces are the weakest IMF, dipole-dipole are stronger but not as strong as Hydrogen bo ...
SBI4U1.1Chemistry of Life
... Aldehydes if the carbonyl group is at the end of the carbon skeleton ...
... Aldehydes if the carbonyl group is at the end of the carbon skeleton ...
Document
... 30. The rate law for the reaction 2NO2 + O3 ––> N2O5 + O2 is rate = k[NO2][O3]. Which one of the following mechanisms is consistent with this rate law? A. NO2 + O3 ––> NO3 + O2 (slow) NO3 + NO2 ––> N2O5 (fast) B. NO2 + NO2 ––> N2O2 + O2 (slow) N2O2 + O3 ––> N2O5 (fast) C. NO2 + O3 ––> NO5 (fast) NO5 ...
... 30. The rate law for the reaction 2NO2 + O3 ––> N2O5 + O2 is rate = k[NO2][O3]. Which one of the following mechanisms is consistent with this rate law? A. NO2 + O3 ––> NO3 + O2 (slow) NO3 + NO2 ––> N2O5 (fast) B. NO2 + NO2 ––> N2O2 + O2 (slow) N2O2 + O3 ––> N2O5 (fast) C. NO2 + O3 ––> NO5 (fast) NO5 ...
Lecture 17-edited
... Hydrogen is the most abundant chemical element in the universe with atomic number 1 and symbol H. It has three isotopes hydrogen 1H, deuterium 2D and tritium 3T and the 1H is the most abundant (99.98%). The hydrogen 1H and deuterium 2D are stable isotopes whereas the tritium ...
... Hydrogen is the most abundant chemical element in the universe with atomic number 1 and symbol H. It has three isotopes hydrogen 1H, deuterium 2D and tritium 3T and the 1H is the most abundant (99.98%). The hydrogen 1H and deuterium 2D are stable isotopes whereas the tritium ...
Organic molecules with functional groups containing oxygen
... fertilizer (natural gas (CH4) is one of the raw materials used in its manufacture), energy used (machinery/transport/ ...
... fertilizer (natural gas (CH4) is one of the raw materials used in its manufacture), energy used (machinery/transport/ ...
Slide 1
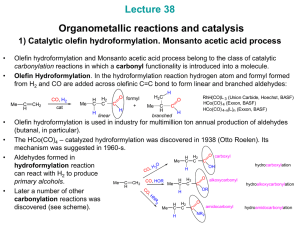
... Co – phosphine modified catalysts. Studies performed at Shell showed that addition of trialkylphosphine ligands changes dramatically the reaction rate and selectivity. When HCo(CO)3(PR3) forms, Co-CO bonds become much stronger and it becomes possible to decrease CO pressure without causing catalyst ...
... Co – phosphine modified catalysts. Studies performed at Shell showed that addition of trialkylphosphine ligands changes dramatically the reaction rate and selectivity. When HCo(CO)3(PR3) forms, Co-CO bonds become much stronger and it becomes possible to decrease CO pressure without causing catalyst ...
• Pergamon
... A variety of substituted 2-methylpyrroles underwent allylic oxidation with the perchlorinated metalloporphyrin 2 and iodosylbenzene in 'IFAlCH2CI2 (9:1). Subsequent addition of an a-free pyrrole to the same reaction mixture afforded an efficient one-pot route to dipyrromethanes. ...
... A variety of substituted 2-methylpyrroles underwent allylic oxidation with the perchlorinated metalloporphyrin 2 and iodosylbenzene in 'IFAlCH2CI2 (9:1). Subsequent addition of an a-free pyrrole to the same reaction mixture afforded an efficient one-pot route to dipyrromethanes. ...
Microsoft Word
... The epoxide ring of the glycidate ester 25a was found to hydrolyze readily in aqueous buffers (pH 6 to 7.5) and this necessitated the use of a waterimmiscible solvent like diisopropyl ether or toluene for the reaction. In the past few years, the use of lipases immobilized in gelatin organo-gels (gel ...
... The epoxide ring of the glycidate ester 25a was found to hydrolyze readily in aqueous buffers (pH 6 to 7.5) and this necessitated the use of a waterimmiscible solvent like diisopropyl ether or toluene for the reaction. In the past few years, the use of lipases immobilized in gelatin organo-gels (gel ...
print
... ring opens up affording a single chain with two carbonyls at the carbons where the double bonds were originally. • Oxidative cleavage is a valuable tool in structure determination, helping to pinpoint the location of double bonds in complex alkene structures. ...
... ring opens up affording a single chain with two carbonyls at the carbons where the double bonds were originally. • Oxidative cleavage is a valuable tool in structure determination, helping to pinpoint the location of double bonds in complex alkene structures. ...
3_2: More Chemical Changes
... • Make a decorative poster explaining 4-6 signs that show a chemical reaction has taken place. • The top 3 signs will be laminated and displayed in the classroom to help you during labs and quizzes • See Ms. B if you need paper, markers, etc. to take home for the night. • DUE MONDAY/TUESDAY ...
... • Make a decorative poster explaining 4-6 signs that show a chemical reaction has taken place. • The top 3 signs will be laminated and displayed in the classroom to help you during labs and quizzes • See Ms. B if you need paper, markers, etc. to take home for the night. • DUE MONDAY/TUESDAY ...
CHAPTER 1: ORGANIC COMPOUNDS
... - C-O-C bond is v-shaped and polar, so molecule is more polar than an alkane with the same number of C’s but not as polar as alcohols with the O-H bond - see table 2 p 46 - can dissolve both polar and non-polar substances - C-O bond stable so they are unrreactive Naming Ethers: - use “-oxy” on end o ...
... - C-O-C bond is v-shaped and polar, so molecule is more polar than an alkane with the same number of C’s but not as polar as alcohols with the O-H bond - see table 2 p 46 - can dissolve both polar and non-polar substances - C-O bond stable so they are unrreactive Naming Ethers: - use “-oxy” on end o ...
Year 13 Organic Chemistry Test
... A second alcohol, 2-methyl propan-2-ol, will not give a positive breathalyzer test. Why not? _____________________________________________________ _____________________________________________________ ...
... A second alcohol, 2-methyl propan-2-ol, will not give a positive breathalyzer test. Why not? _____________________________________________________ _____________________________________________________ ...
Exam 2 - Wake Forest University
... Do not open or begin this exam until instructed. This exam consists of 5 pages plus the cover page. Before starting the exam, check to make sure that you have all of the pages. The exam has a total of 100 points and includes 13 questions. Only legible answers written on the exam will be considered f ...
... Do not open or begin this exam until instructed. This exam consists of 5 pages plus the cover page. Before starting the exam, check to make sure that you have all of the pages. The exam has a total of 100 points and includes 13 questions. Only legible answers written on the exam will be considered f ...
AP Chemistry - Dorman High School
... Butane and above can exhibit isomerism See Figure (butane and isobutene) ...
... Butane and above can exhibit isomerism See Figure (butane and isobutene) ...
2 - CronScience
... 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O2-, is –½. What are the oxidation numbers of all the elements in HCO3- ? ...
... 6. The sum of the oxidation numbers of all the atoms in a molecule or ion is equal to the charge on the molecule or ion. 7. Oxidation numbers do not have to be integers. Oxidation number of oxygen in the superoxide ion, O2-, is –½. What are the oxidation numbers of all the elements in HCO3- ? ...
Nucleophilic Aromatic Substitution, General Corrected Mechanism
... Nucleophilic aromatic substitution in electron-deficient arenas, particularly nitroarenes is an efficient tool in synthesis and manufacturing of pharmaceuticals [1-3]. It is therefore of crucial importance to know how exactly these reactions proceed. For many years it was considered that these react ...
... Nucleophilic aromatic substitution in electron-deficient arenas, particularly nitroarenes is an efficient tool in synthesis and manufacturing of pharmaceuticals [1-3]. It is therefore of crucial importance to know how exactly these reactions proceed. For many years it was considered that these react ...
Precipitate Lab Report Power Point with Answers
... Temperature change, odor change, precipitate formation, irreversibility, color change, and new bubble formation are the evidence for a chemical reaction occuring. Not every time one of these changes is proof of a chemical reaction, but often they are. Sometimes chemical reactions can occur with no o ...
... Temperature change, odor change, precipitate formation, irreversibility, color change, and new bubble formation are the evidence for a chemical reaction occuring. Not every time one of these changes is proof of a chemical reaction, but often they are. Sometimes chemical reactions can occur with no o ...
Chapter 13. Plannig and Execution of Multistep Synthesis
... vinyl ether in dihydropyran. (catalyst: p-toluenesulfonic acid or its pyridinium salt) ...
... vinyl ether in dihydropyran. (catalyst: p-toluenesulfonic acid or its pyridinium salt) ...
Asymmetric induction

Asymmetric induction (also enantioinduction) in stereochemistry describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis.Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist.Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst of chiral ligand. This method of asymmetric synthesis is economically most desirable.