
Mechanistic Studies on Alcoholysis of α-Keto esters
... form hydrates and hemiacetals of keto carbonyls in the presence of water and alcohols, respectively.3 In the course of our studies on the solvent effect in photochemistry of phenacyl carboxylates, we had a chance to prepare and work with 2-methylphenacyl phenylglyoxylate (1a), which we found it very ...
... form hydrates and hemiacetals of keto carbonyls in the presence of water and alcohols, respectively.3 In the course of our studies on the solvent effect in photochemistry of phenacyl carboxylates, we had a chance to prepare and work with 2-methylphenacyl phenylglyoxylate (1a), which we found it very ...
Chemistry in Action: Question paper - A
... 1 Sodium thiosulfate solution (Na2S2O3) reacts slowly with dilute hydrochloric acid to form a precipitate. The rate of this reaction can be studied by measuring the time (t) that it takes for a small fixed amount of precipitate to form under different conditions. The fixed amount of precipitate is t ...
... 1 Sodium thiosulfate solution (Na2S2O3) reacts slowly with dilute hydrochloric acid to form a precipitate. The rate of this reaction can be studied by measuring the time (t) that it takes for a small fixed amount of precipitate to form under different conditions. The fixed amount of precipitate is t ...
Document
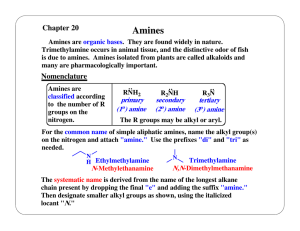
... Many are chiral and occur in single enantiomer form. Some of these compounds are pharmacologically important, e. g., quinine and atropine. Others have dangerous natures, e. g., morphine and strychnine. Some alkaloids have been used to separate the enantiomers of chiral carboxylic acids. This resolut ...
... Many are chiral and occur in single enantiomer form. Some of these compounds are pharmacologically important, e. g., quinine and atropine. Others have dangerous natures, e. g., morphine and strychnine. Some alkaloids have been used to separate the enantiomers of chiral carboxylic acids. This resolut ...
Chapter 9
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
... Fluorine is too reactive to give mono-fluorinated products For Iodine, an oxidizing agent such as hydrogen peroxide or a copper salt such as CuCl2 must be added to the reaction • These substances oxidize I2 to the electrophilic species that reacts as if it were I+ • The aromatic ring reacts with the ...
Synthetic Applications of Zinc Borohydride
... Chiral amino alcohols are useful in, among others, asymmetric synthesis,22 peptide and pharmaceutical chemistry,23 and the synthesis of insecticidal compounds.24 Earlier preparative methods used reduction of esters of amino acids by sodium in ethanol.25 Subsequently, LiAlH426 and NaBH427 were used f ...
... Chiral amino alcohols are useful in, among others, asymmetric synthesis,22 peptide and pharmaceutical chemistry,23 and the synthesis of insecticidal compounds.24 Earlier preparative methods used reduction of esters of amino acids by sodium in ethanol.25 Subsequently, LiAlH426 and NaBH427 were used f ...
Common aldehydes and ketones
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
... • A compound containing a carbonyl group (C=O) is normally in rapid equilibrium with an enol tautomer, which contains a pair of doubly bonded carbon atoms adjacent to a hydroxyl (−OH) group, C=C-OH. The keto form predominates at equilibrium for most ketones. Nonetheless, the enol form is important f ...
12 - Wiley
... 12 Fischer Esterification • Fischer esterification is an equilibrium reaction • by careful control of experimental conditions, it is possible to prepare esters in high yield • if the alcohol is inexpensive relative to the carboxylic acid, it can be used in excess to drive the equilibrium to the rig ...
... 12 Fischer Esterification • Fischer esterification is an equilibrium reaction • by careful control of experimental conditions, it is possible to prepare esters in high yield • if the alcohol is inexpensive relative to the carboxylic acid, it can be used in excess to drive the equilibrium to the rig ...
06_chapter 1
... Sarett56 for the oxidation of steroidal alcohols. The oxidant is taken in 3:1 molar ratio with the substrate at room temperature. The yield of ketones is about 90 percent. Acid sensitive functional groups like acetal groups as well as double bonds and thioethers are not affected by the reagent. Pyri ...
... Sarett56 for the oxidation of steroidal alcohols. The oxidant is taken in 3:1 molar ratio with the substrate at room temperature. The yield of ketones is about 90 percent. Acid sensitive functional groups like acetal groups as well as double bonds and thioethers are not affected by the reagent. Pyri ...
Bk2P06EE
... is directly proportional to the number of moles of gaseous molecules, the forward reaction with less gaseous molecules would be favoured by high pressure. Hence, in order to increase the yield of ammonia, the process is carried out at a high pressure. ...
... is directly proportional to the number of moles of gaseous molecules, the forward reaction with less gaseous molecules would be favoured by high pressure. Hence, in order to increase the yield of ammonia, the process is carried out at a high pressure. ...
Bioorthoganal Chemistry to Selectively Label Cellular Proteins
... Carboxylate residues react with carbodiimides. Causes formation of active esters Can go on to react amines, or undergo O‐ acylisouronium rearrangement M. B. Francis in Chemical Biology Wiley‐VCH, Weinheim, 2007, p. 593 ...
... Carboxylate residues react with carbodiimides. Causes formation of active esters Can go on to react amines, or undergo O‐ acylisouronium rearrangement M. B. Francis in Chemical Biology Wiley‐VCH, Weinheim, 2007, p. 593 ...
vce chemistry trial exam 1
... A is incorrect because the components have different areas under the peaks, which indicates they are present in different quantities. The second peak has approximately twice the area under the peak compared to the first peak, so is present in twice the amount of the other. C is incorrect because onl ...
... A is incorrect because the components have different areas under the peaks, which indicates they are present in different quantities. The second peak has approximately twice the area under the peak compared to the first peak, so is present in twice the amount of the other. C is incorrect because onl ...
KAZAKH-TURKISH HIGH SCHOOLS
... 1- Calculate the percent by mass of the solute in each of the following solutions. a) 15 g NaCl in 360 g water b) 40 g sugar in 762 ml of solution with a density of 1.05 g/ml 2- What is the molarity of a 85 ml ethanol (C2H5OH) solution containing 1.77 g of ethanol ? 3- The 0.5 M , 200 ml of MgCl2 so ...
... 1- Calculate the percent by mass of the solute in each of the following solutions. a) 15 g NaCl in 360 g water b) 40 g sugar in 762 ml of solution with a density of 1.05 g/ml 2- What is the molarity of a 85 ml ethanol (C2H5OH) solution containing 1.77 g of ethanol ? 3- The 0.5 M , 200 ml of MgCl2 so ...
LDA preparation and other lab techniques
... should start with a clear organic layer (washed with brine, and not poured into a wet flask). Drying takes longer than for magnesium sulfate. Sit it over the drying agent ~15 minutes for dichloromethane, ~30 minutes for ethyl acetate. It does not work well for ether. Celite: Sometimes it is useful t ...
... should start with a clear organic layer (washed with brine, and not poured into a wet flask). Drying takes longer than for magnesium sulfate. Sit it over the drying agent ~15 minutes for dichloromethane, ~30 minutes for ethyl acetate. It does not work well for ether. Celite: Sometimes it is useful t ...
Reaction Yields
... greater than the stoichiometric ratio of 1:1. Hydrogen, therefore, is present in excess, and chlorine is the limiting reactant. Reaction of all the provided chlorine (2 mol) will consume 2 mol of the 3 mol of hydrogen provided, leaving 1 mol of hydrogen unreacted. An alternative approach to identify ...
... greater than the stoichiometric ratio of 1:1. Hydrogen, therefore, is present in excess, and chlorine is the limiting reactant. Reaction of all the provided chlorine (2 mol) will consume 2 mol of the 3 mol of hydrogen provided, leaving 1 mol of hydrogen unreacted. An alternative approach to identify ...
Cl3CCN/PPh3 and CBr4/PPh3: two efficient reagent systems for the
... in a 94% yield, whereas none of the expected bromide was obtained (entries 531 and 632). 3-Hydroxypyridine and 8-hydroxyquinoline gave no halo-product (entries 333, 434, 935 and 1036). The reaction also proceeded with hydroxyquinazolines, however, the conditions needed to be modified slightly. Since ...
... in a 94% yield, whereas none of the expected bromide was obtained (entries 531 and 632). 3-Hydroxypyridine and 8-hydroxyquinoline gave no halo-product (entries 333, 434, 935 and 1036). The reaction also proceeded with hydroxyquinazolines, however, the conditions needed to be modified slightly. Since ...
chapter27
... Alcohols and Phenols • The functional group in alcohols and phenols is the hydroxyl (-OH) group. • Alcohols and phenols can be considered derivatives of hydrocarbons in which one or more H atoms have been replaced by -OH groups. • Phenols are derivatives of benzene in which one H has been replaced ...
... Alcohols and Phenols • The functional group in alcohols and phenols is the hydroxyl (-OH) group. • Alcohols and phenols can be considered derivatives of hydrocarbons in which one or more H atoms have been replaced by -OH groups. • Phenols are derivatives of benzene in which one H has been replaced ...
Carbon-Carbon Bond Formation by Reductive
... couplings, that of diphenylacetylene to yield exclusively (E,E)-1,2,3,4-tetraphenyl-l,3-buta diene and that of acetophenone to produce only racem/c-2,3-diphenyl-2,3-butanediol, is inter preted to conclude that the couplings proceed via two electron transfer pathways (TET) involving titanium (IV ) ...
... couplings, that of diphenylacetylene to yield exclusively (E,E)-1,2,3,4-tetraphenyl-l,3-buta diene and that of acetophenone to produce only racem/c-2,3-diphenyl-2,3-butanediol, is inter preted to conclude that the couplings proceed via two electron transfer pathways (TET) involving titanium (IV ) ...
Topic Selection Menu - Pennsylvania State University
... Protonation of carbonyl, resonance forms Nucleophile attack Tetrahedral intermediate Deprotonation - protonation ...
... Protonation of carbonyl, resonance forms Nucleophile attack Tetrahedral intermediate Deprotonation - protonation ...
Topic Selection Menu - Pennsylvania State University
... Protonation of carbonyl, resonance forms Nucleophile attack Tetrahedral intermediate Deprotonation - protonation ...
... Protonation of carbonyl, resonance forms Nucleophile attack Tetrahedral intermediate Deprotonation - protonation ...
questionsheet 1 e/z (cis/trans) isomerism
... Mix / react ethanoyl chloride and the amine at room temperature (1) Recrystallise the product (1) ...
... Mix / react ethanoyl chloride and the amine at room temperature (1) Recrystallise the product (1) ...
BIOLOGY and CHEMISTRY - The set of Entrance Exam Questions
... b. individuals of several types of species in a specifically defined area c. all the plants and animals on Earth, living at the same time d. all the individuals of one species on Earth 62. Viruses can be observed: a. by light microscopy at 1000x enlargement b. only by electron microscopy c. by light ...
... b. individuals of several types of species in a specifically defined area c. all the plants and animals on Earth, living at the same time d. all the individuals of one species on Earth 62. Viruses can be observed: a. by light microscopy at 1000x enlargement b. only by electron microscopy c. by light ...
Reduction Reactions
... A number of methods have been developed for forming the anti-1,3-diol from the corresponding chiral 2-hydroxy-ketone. All rely on the so-called DIRECTED REDUCTION which takes advantage of the intramolecular hydride transfer through a well-defined 6membered chair-like transition state (compare: Meerw ...
... A number of methods have been developed for forming the anti-1,3-diol from the corresponding chiral 2-hydroxy-ketone. All rely on the so-called DIRECTED REDUCTION which takes advantage of the intramolecular hydride transfer through a well-defined 6membered chair-like transition state (compare: Meerw ...
Petasis reaction

The Petasis reaction (alternatively called the Petasis borono–Mannich (PBM) reaction) is the chemical reaction of an amine, aldehyde, and vinyl- or aryl-boronic acid to form substituted amines.Reported in 1993 by Nicos Petasis as a practical method towards the synthesis of a geometrically pure antifungal agent, naftifine, the Petasis reaction can be described as a variation of the Mannich reaction. Rather than generating an enolate to form the substituted amine product, in the Petasis reaction, the vinyl group of the organoboronic acid serves as the nucleophile. In comparison to other methods of generating allyl amines, the Petasis reaction tolerates a multifunctional scaffold, with a variety of amines and organoboronic acids as potential starting materials. Additionally, the reaction does not require anhydrous or inert conditions. As a mild, selective synthesis, the Petasis reaction is useful in generating α-amino acids, and is utilized in combinatorial chemistry and drug discovery.




![Neutral ionic liquid [BMIm]BF4 promoted highly selective](http://s1.studyres.com/store/data/017897985_1-047f9869d5604c115b21339541ccfffe-300x300.png)

















