Chapter 9. Addition Reactions of Alkenes
... The reaction below, which provides compound M as its major product, appears to defy the principles that we discussed in class. Draw the structures of the intermediate carbocations that form in this reaction, then clearly but briefly explain why M, and not L, is the major product of this reaction. Hi ...
... The reaction below, which provides compound M as its major product, appears to defy the principles that we discussed in class. Draw the structures of the intermediate carbocations that form in this reaction, then clearly but briefly explain why M, and not L, is the major product of this reaction. Hi ...
LIMITING REACTANT LAB
... For the quiz on Thursday, turn in PS D2 – D7. Staple them together in sequential order (D2 first). Plus turn in your lab book as I will be checking your write up of the limiting reaction lab (make sure you recorded your data and answered the questions). Items to review for the quiz: 1. Naming (Chp. ...
... For the quiz on Thursday, turn in PS D2 – D7. Staple them together in sequential order (D2 first). Plus turn in your lab book as I will be checking your write up of the limiting reaction lab (make sure you recorded your data and answered the questions). Items to review for the quiz: 1. Naming (Chp. ...
Chem 30B Spring 2004 QUIZ #1 KEY Weds April 14th / 30
... 6. What is the correct order of boiling points from LOWEST to HIGHEST of the following alcohols? ...
... 6. What is the correct order of boiling points from LOWEST to HIGHEST of the following alcohols? ...
3.8 Aldehydes and ketones
... Oxygen is more electronegative than carbon meaning that the p electrons will be highly distorted towards the oxygen atom as shown above. ...
... Oxygen is more electronegative than carbon meaning that the p electrons will be highly distorted towards the oxygen atom as shown above. ...
review sheet plus practice problems
... What are the products of free radical halogenation of an alkane (ex: Cl2/uv light)? Give the chain mechanism for free radical halogenation. What is the selectivity for brominations vs. fluorinations? Why are allylic radicals more stable than other types? Draw resonance for all allylic radicals. What ...
... What are the products of free radical halogenation of an alkane (ex: Cl2/uv light)? Give the chain mechanism for free radical halogenation. What is the selectivity for brominations vs. fluorinations? Why are allylic radicals more stable than other types? Draw resonance for all allylic radicals. What ...
Chapter 17: Aldehydes and Ketones: Nucleophilic Addition to the
... Spectra of Aldehydes and Ketones: The 1H chemical shift range for the aldehyde proton is δ 9-10 ppm The aldehyde proton will couple to the protons on the α-carbon with a typical coupling constant of J ≈ 2 Hz A carbonyl will slightly deshield the protons on the α-carbon; typical chemical shift range ...
... Spectra of Aldehydes and Ketones: The 1H chemical shift range for the aldehyde proton is δ 9-10 ppm The aldehyde proton will couple to the protons on the α-carbon with a typical coupling constant of J ≈ 2 Hz A carbonyl will slightly deshield the protons on the α-carbon; typical chemical shift range ...
CARBONYL COMPOUNDS
... and HCHO (MR = 30) and put these compounds in order with respect to increasing boiling point. C2H6 CH3OH HCHO Methanal is a gas, other important carbonyl compounds are …………………... Early members are soluble in water due to ……………….. ………………. between hydrogen from water and oxygen from the carboxylic gro ...
... and HCHO (MR = 30) and put these compounds in order with respect to increasing boiling point. C2H6 CH3OH HCHO Methanal is a gas, other important carbonyl compounds are …………………... Early members are soluble in water due to ……………….. ………………. between hydrogen from water and oxygen from the carboxylic gro ...
i m. pharm. - Rajiv Gandhi University of Health Sciences
... the practical pyruvic acid production was 42.4 g/L. The determination coefficient (R2) was 0.9483, which ensures adequate credibility of the model. 8. Chanakya Pallem et al 12., carried out solid state fermentation (SSF) for the production of L-glutaminase by the fungal strain Trichoderma koningii ...
... the practical pyruvic acid production was 42.4 g/L. The determination coefficient (R2) was 0.9483, which ensures adequate credibility of the model. 8. Chanakya Pallem et al 12., carried out solid state fermentation (SSF) for the production of L-glutaminase by the fungal strain Trichoderma koningii ...
Workshop 5
... with CH4 and Cl2. Explain why lower temperatures are needed for the halogenation reaction using (CH3)4Pb as the initiator than with Cl2 using light or heat. b. An alternative to the accepted mechanism for the propagation steps in the chlorination of methane is given below. ...
... with CH4 and Cl2. Explain why lower temperatures are needed for the halogenation reaction using (CH3)4Pb as the initiator than with Cl2 using light or heat. b. An alternative to the accepted mechanism for the propagation steps in the chlorination of methane is given below. ...
2015 CH 420 Take Home Quiz 3 March 24
... could “flip” either substrate horizontally to see the other theoretical product). In the boxes above, draw an arrow to the more nucleophilic nitrogen atom in the hydrazine substrate. In addition, circle the most electrophilic carbonyl in the 1,3-diketone substrate. ...
... could “flip” either substrate horizontally to see the other theoretical product). In the boxes above, draw an arrow to the more nucleophilic nitrogen atom in the hydrazine substrate. In addition, circle the most electrophilic carbonyl in the 1,3-diketone substrate. ...
Functional Groups PP
... only)? Which is an alkene (double bond)? An alkyne (triple bond)? 1. C2H6 2. C2H4 3. C2H2 ...
... only)? Which is an alkene (double bond)? An alkyne (triple bond)? 1. C2H6 2. C2H4 3. C2H2 ...
Acyl Anions Derived from Enol Ethers
... The a-hydrogens of nitroalkanes are appreciably acidic due to resonance stabilization of the anion [CH3N02, pKa: 10.2; CH3CH2N02, pKa: 8.51. The anions derived from nitroalkanes give typical nucleophilic addition reactions with aldehydes (the Henry-Nef tandem reaction). Note that the nitro group can ...
... The a-hydrogens of nitroalkanes are appreciably acidic due to resonance stabilization of the anion [CH3N02, pKa: 10.2; CH3CH2N02, pKa: 8.51. The anions derived from nitroalkanes give typical nucleophilic addition reactions with aldehydes (the Henry-Nef tandem reaction). Note that the nitro group can ...
Background Information
... The Lucas reagent is an aqueous solution of strong acid (HCl) and zinc chloride. An insoluble layer, cloudiness, color change (red or orange) or an emulsion will form with 1°, 2°, 3° allylic, 3° alkyl and some 2 ° alcohols and constitutes a “positive” result. Students should compare his/her results ...
... The Lucas reagent is an aqueous solution of strong acid (HCl) and zinc chloride. An insoluble layer, cloudiness, color change (red or orange) or an emulsion will form with 1°, 2°, 3° allylic, 3° alkyl and some 2 ° alcohols and constitutes a “positive” result. Students should compare his/her results ...
Chem 30BL * Lecture 2 - UCLA Chemistry and Biochemistry
... • Assemble the setup as shown in the picture. An O-ring has to be placed below the compression cap! • Place the cyclohexanol, the Montmorillonite K10 and a properly placed spin vane in the conical vial • If the conical vial and the Al-block have poor contact, use Al-foil on the sides and the bottom ...
... • Assemble the setup as shown in the picture. An O-ring has to be placed below the compression cap! • Place the cyclohexanol, the Montmorillonite K10 and a properly placed spin vane in the conical vial • If the conical vial and the Al-block have poor contact, use Al-foil on the sides and the bottom ...
ClickHere - KV HVF , AVADI Chennai
... is 1500Ω. What is the cell constant, if the conductivity of 0.001 M KCl solution at ...
... is 1500Ω. What is the cell constant, if the conductivity of 0.001 M KCl solution at ...
Chem 30BL_Lecture 2_.. - UCLA Chemistry and Biochemistry
... • Assemble the setup as shown in the picture. An O-ring has to be placed below the compression cap! • Place the cyclohexanol, the Montmorillonite K10 and a properly placed spin vane in the conical vial • If the conical vial and the Al-block have poor contact, use Al-foil on the sides and the bottom ...
... • Assemble the setup as shown in the picture. An O-ring has to be placed below the compression cap! • Place the cyclohexanol, the Montmorillonite K10 and a properly placed spin vane in the conical vial • If the conical vial and the Al-block have poor contact, use Al-foil on the sides and the bottom ...
Document
... Diazomethane forms methylene, which converts alkenes into cyclopropanes. The highly reactive species methylene, H2C: (the simplest carbene) can be produced from the decomposition of diazomethane: ...
... Diazomethane forms methylene, which converts alkenes into cyclopropanes. The highly reactive species methylene, H2C: (the simplest carbene) can be produced from the decomposition of diazomethane: ...
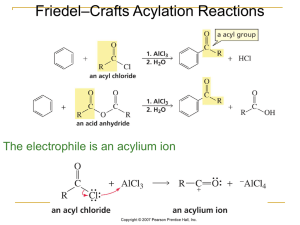
Slide 1
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation ...
... It is not possible to obtain a good yield of an alkylbenzene containing a straight-chain group via Friedel–Crafts alkylation due to carbocation ...
Chapter 16 Aldehydes and Ketones I. Nucleophilic Addition to
... Dissolving aldehydes or ketones in water causes formation of an equilibrium between the carbonyl compound and its hydrate l The hydrate is also called a gem-diol l The equilibrum favors a ketone over its hydrate because the tetrahedral ketone hydrate is stericallycrowded l Aqueous solution of formal ...
... Dissolving aldehydes or ketones in water causes formation of an equilibrium between the carbonyl compound and its hydrate l The hydrate is also called a gem-diol l The equilibrum favors a ketone over its hydrate because the tetrahedral ketone hydrate is stericallycrowded l Aqueous solution of formal ...
اســـم المـــدرس: د
... 2) Write the chemical and the reaction mechanism for the reaction of benzaldehyde with excess methanol and acid catalyst. ...
... 2) Write the chemical and the reaction mechanism for the reaction of benzaldehyde with excess methanol and acid catalyst. ...
16.7 Addition of Alcohols: Hemiacetals and Acetals
... • The carbonyl group is a C=O. The group is polar, with a partial (-) charge on O and a partial (+) charge on C. The O and the two substituents on the carbonyl-group C atom form a planar triangle. • The simplest aldehydes and ketones are known by common names. Aldehydes are named systematically by r ...
... • The carbonyl group is a C=O. The group is polar, with a partial (-) charge on O and a partial (+) charge on C. The O and the two substituents on the carbonyl-group C atom form a planar triangle. • The simplest aldehydes and ketones are known by common names. Aldehydes are named systematically by r ...
Synthesis of a Family of Chiral Asymmetric Schiff - Blogs at H-SC
... The Baylis-Hillman reaction, patented in 1972, is a novel condensation of an acrylate ester and an aldehyde resulting in a β-hydroxy-αmethylene ester. This reaction is particularly interesting to synthetic chemists because the product is densely functionalized and the reaction can be stereospecific ...
... The Baylis-Hillman reaction, patented in 1972, is a novel condensation of an acrylate ester and an aldehyde resulting in a β-hydroxy-αmethylene ester. This reaction is particularly interesting to synthetic chemists because the product is densely functionalized and the reaction can be stereospecific ...
CARBONYL COMPOUNDS
... Formation of carbonyl compounds from alcohols Aldehydes • Oxidation of primary (1°) alcohols - risk of oxidation to acids ...
... Formation of carbonyl compounds from alcohols Aldehydes • Oxidation of primary (1°) alcohols - risk of oxidation to acids ...
Chapter One: Molecular Structure
... reaction between ethers and epoxides with nucleophiles under acidic and basic conditions. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target ether or epoxide in high yi ...
... reaction between ethers and epoxides with nucleophiles under acidic and basic conditions. Predict the stereochemistry and optical activity of a product from an understanding of its mechanism of formation. Propose a reaction or sequence of reactions to produce a target ether or epoxide in high yi ...
Wolff–Kishner reduction

The Wolff–Kishner reduction is a reaction used in organic chemistry to convert carbonyl functionalities into methylene groups. In the context of complex molecule synthesis, it is most frequently employed to remove a carbonyl group after it has served its synthetic purpose of activating an intermediate in a preceding step. As such, there is no obvious retron for this reaction. Originally reported by Nikolai Kischner in 1911 and Ludwig Wolff in 1912, it has been applied to the total synthesis of scopadulcic acid B, aspidospermidine and dysidiolide.In general, the reaction mechanism first involves the in situ generation of a hydrazone by condensation of hydrazine with the ketone or aldehyde substrate. Sometimes it is however advantageous to use a pre-formed hydrazone as substrate (see modifications). The hydrazone is deprotonated by alkoxide base followed by a concerted, rate-determining step in which a diimide anion is formed. Collapse of this alkyldiimde with loss of N2 leads to formation of an alkylanion which can be protonated by solvent to give the desired product.Because the Wolff–Kishner reduction requires highly basic conditions, it is unsuitable for base-sensitive substrates. However, this method can be superior over the related Clemmensen reduction for acid-sensitive compounds such as pyrroles and for high-molecular weight compounds.