Chemdraw B&W - Pennsylvania State University
... carbonyl functional group (C=O) • The compounds occur widely in nature as intermediates in metabolism and biosynthesis • They are also common as chemicals, as solvents, monomers, adhesives, agrichemicals and pharmaceuticals ...
... carbonyl functional group (C=O) • The compounds occur widely in nature as intermediates in metabolism and biosynthesis • They are also common as chemicals, as solvents, monomers, adhesives, agrichemicals and pharmaceuticals ...
C h e m g u id e –... ACYL CHLORIDES: REACTIONS WITH WATER, ALCOHOLS AND PHENOLS
... group. Ethanoylation is a particular example of acylation where a CH3C=O group (an ethanoyl group) is substituted into another molecule. ...
... group. Ethanoylation is a particular example of acylation where a CH3C=O group (an ethanoyl group) is substituted into another molecule. ...
Alcohols, Phenols, and Ethers
... a) All three statements are true. b) Two of the three statements are true. c) Only one of the statements is true. d) None of the statements is true. 14.38 c - FTF Statements: (1) Glycerin, a three-carbon diol, has a great affinity for moisture (water vapor). (2) Oxidation of a thiol produces a disul ...
... a) All three statements are true. b) Two of the three statements are true. c) Only one of the statements is true. d) None of the statements is true. 14.38 c - FTF Statements: (1) Glycerin, a three-carbon diol, has a great affinity for moisture (water vapor). (2) Oxidation of a thiol produces a disul ...
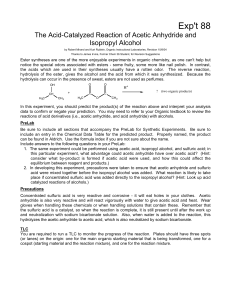
The Acid-Catalyzed Reaction of Acetic
... Ester syntheses are one of the more enjoyable experiments in organic chemistry, as one can't help but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, ...
... Ester syntheses are one of the more enjoyable experiments in organic chemistry, as one can't help but notice the special odors associated with esters - some fruity, some more like nail polish. In contrast, the acids which are used in their syntheses usually have a rotten odor. The reverse reaction, ...
Project Overview
... the temperature at which an equilibrium exists between the wellordered crystalline state and the more ...
... the temperature at which an equilibrium exists between the wellordered crystalline state and the more ...
Solution 1. - TutorBreeze.com
... is the product of aldol condensation, in which two molecules of aldehydes , or two molecules of ketones or one molecule of aldehyde and one molecule of ketone condense in the presence of aqueos sodium hydroxide to form aldol. ...
... is the product of aldol condensation, in which two molecules of aldehydes , or two molecules of ketones or one molecule of aldehyde and one molecule of ketone condense in the presence of aqueos sodium hydroxide to form aldol. ...
carbonyl group
... Aldehydes and ketones are characterized by the presence of the carbonyl group, which is perhaps the most important functional group in organic chemistry. Aldehydes have at least one hydrogen atom attached to the carbonyl carbon atom. The remaining group may be another hydrogen atom or any aliphatic ...
... Aldehydes and ketones are characterized by the presence of the carbonyl group, which is perhaps the most important functional group in organic chemistry. Aldehydes have at least one hydrogen atom attached to the carbonyl carbon atom. The remaining group may be another hydrogen atom or any aliphatic ...
Organic Chemistry PPT including assignments File
... hydrocarbon that we have talked about: alkane, alkene, alkyne, alcohol, carboxylic acids, amines, esters In the chart, include ...
... hydrocarbon that we have talked about: alkane, alkene, alkyne, alcohol, carboxylic acids, amines, esters In the chart, include ...
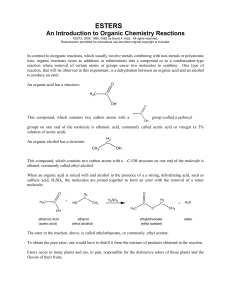
Esters - chymist.com
... In contrast to inorganic reactions, which usually involve metals combining with non-metals or polyatomic ions, organic reactions occur as additions or substitutions into a compound or as a condensation-type reaction where removal of certain atoms or groups cause two molecules to combine. One type of ...
... In contrast to inorganic reactions, which usually involve metals combining with non-metals or polyatomic ions, organic reactions occur as additions or substitutions into a compound or as a condensation-type reaction where removal of certain atoms or groups cause two molecules to combine. One type of ...
Descriptive Chemistry for the Final Exam
... There are several other allotropes of carbon. Buckminster fullerene C60 and carbon nanotubes are the most famous of the recently discovered allotropes. They contain 5 and 6 member rings of carbon atoms. Forms covalent compounds with non-metals mostly with a valence of four. It is the basis for organ ...
... There are several other allotropes of carbon. Buckminster fullerene C60 and carbon nanotubes are the most famous of the recently discovered allotropes. They contain 5 and 6 member rings of carbon atoms. Forms covalent compounds with non-metals mostly with a valence of four. It is the basis for organ ...
Chapter 10
... when a carbon or hydrogen that is connected to a carbon atom in a structure is replaced by oxygen, nitrogen, or halogen Not defined as loss of electrons by an atom as in ...
... when a carbon or hydrogen that is connected to a carbon atom in a structure is replaced by oxygen, nitrogen, or halogen Not defined as loss of electrons by an atom as in ...
sample paper - CBSE PORTAL
... Q.1 Excess of potassium makes KCl crystals lilac, Why ? Q.2 People living at high altitude generally suffer from anoxia , Explain. Q.3 Define limiting molar conductivity . Q.4 “A greater insight in to the energetic and mechanistic aspects of reactions”. Explain the above statement which was given by ...
... Q.1 Excess of potassium makes KCl crystals lilac, Why ? Q.2 People living at high altitude generally suffer from anoxia , Explain. Q.3 Define limiting molar conductivity . Q.4 “A greater insight in to the energetic and mechanistic aspects of reactions”. Explain the above statement which was given by ...
EXPERIMENT 3: Preparation and Reactivity of Alkyl Halides
... both mechanisms is the protonation of the alcohol to form an oxonium ion, converting the OH group into a good leaving group. What happens next depends on the nature of the alkyl group, R. If R is a group that readily forms a carbocation, then the slow, rate-determining step is the loss of a water mo ...
... both mechanisms is the protonation of the alcohol to form an oxonium ion, converting the OH group into a good leaving group. What happens next depends on the nature of the alkyl group, R. If R is a group that readily forms a carbocation, then the slow, rate-determining step is the loss of a water mo ...
Lecture - Ch 17
... • When the 1H NMR spectrum of an alcohol is run in dimethyl sulfoxide solvent rather than in chloroform, exchange of the O–H proton is slow and spin–spin splitting is seen between the O–H proton and C–H protons on the adjacent carbon What spin multiplicities are expected for the hydroxyl protons in ...
... • When the 1H NMR spectrum of an alcohol is run in dimethyl sulfoxide solvent rather than in chloroform, exchange of the O–H proton is slow and spin–spin splitting is seen between the O–H proton and C–H protons on the adjacent carbon What spin multiplicities are expected for the hydroxyl protons in ...
ALDEHYDES , KETONES AND CARBOXYLIC ACIDS
... 2. Reduction: To alcohols (Reducing agents are NaBH4, LiAlH4 andH2/ catalyst) R-CHO +[H]→ R-CH2OH R2CO+ [H] → R2CHOH Reduction to hydrocarbons: Clemmensen reduction: The carbonyl group of Wolff-Kishner reduction: The carbonyl group of aldehydes and ketones is reduced to CH2 group on aldehydes and ke ...
... 2. Reduction: To alcohols (Reducing agents are NaBH4, LiAlH4 andH2/ catalyst) R-CHO +[H]→ R-CH2OH R2CO+ [H] → R2CHOH Reduction to hydrocarbons: Clemmensen reduction: The carbonyl group of Wolff-Kishner reduction: The carbonyl group of aldehydes and ketones is reduced to CH2 group on aldehydes and ke ...
Document
... • There is a different end result here, though as elimination of water occurs. • The initial reaction is attack of the amine on the carbonyl to give the alkoxide intermediate as normal. • Following protonation of the alkoxide and loss of the proton on the amino moiety, an aminol is generated. • The ...
... • There is a different end result here, though as elimination of water occurs. • The initial reaction is attack of the amine on the carbonyl to give the alkoxide intermediate as normal. • Following protonation of the alkoxide and loss of the proton on the amino moiety, an aminol is generated. • The ...
Mock-UP - GEOCITIES.ws
... Carbonyl group to have two strong poles from electronic in π bond, and that to be formed move aside by direction the oxygen atom, that have more electronegative, therefore the carbonyl carbon atom to be formed deficient electron, this property to become Carbonyl carbon is to work on strong the acidi ...
... Carbonyl group to have two strong poles from electronic in π bond, and that to be formed move aside by direction the oxygen atom, that have more electronegative, therefore the carbonyl carbon atom to be formed deficient electron, this property to become Carbonyl carbon is to work on strong the acidi ...
Chemistry 199 - Department of Chemistry | Oregon State University
... What is meant by a condensation reaction? Give an example. Water is lost during a condensation reaction. Examples include: the formation of an ester from an alcohol and a carboxylic acid, the formation of an amide from an amine and a carboxylic acid. ...
... What is meant by a condensation reaction? Give an example. Water is lost during a condensation reaction. Examples include: the formation of an ester from an alcohol and a carboxylic acid, the formation of an amide from an amine and a carboxylic acid. ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.