Chapter 9
... reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. • HBr and HI are strong acids that are also sources of good nucleophiles (Br¯ and I¯, respectively). • When ethers react with HBr or HI, both C-O bonds are cleaved ...
... reactions, their poor leaving group must first be converted into a good leaving group by reaction with strong acids such as HBr and HI. • HBr and HI are strong acids that are also sources of good nucleophiles (Br¯ and I¯, respectively). • When ethers react with HBr or HI, both C-O bonds are cleaved ...
CH 102 Laboratory 7 Ester Synthesis and Smells
... can usually be remedied by using a reaction mixture that does not contain water and by removing the water that is produced by the reaction. Various strategies have been developed to perform this task. For example, concentrated sulfuric acid can be added to the reaction. The sulfuric acid reacts rapi ...
... can usually be remedied by using a reaction mixture that does not contain water and by removing the water that is produced by the reaction. Various strategies have been developed to perform this task. For example, concentrated sulfuric acid can be added to the reaction. The sulfuric acid reacts rapi ...
CH102 Practice exam 2
... ____ 1.Tertiary alcohols are not easily oxidized. ____ 2.Secondary alcohols can be oxidized to aldehydes. ____ 3.Primary alcohols can be oxidized either to aldehydes or carboxylic acids. ____ 4.Alcohols can form either alkenes or ethers upon dehydration. ____ 5.Thiols are easily oxidized to disulfid ...
... ____ 1.Tertiary alcohols are not easily oxidized. ____ 2.Secondary alcohols can be oxidized to aldehydes. ____ 3.Primary alcohols can be oxidized either to aldehydes or carboxylic acids. ____ 4.Alcohols can form either alkenes or ethers upon dehydration. ____ 5.Thiols are easily oxidized to disulfid ...
Functional Groups
... • If the alcohol is attached to a carbon that is also attached to two hydrogens and one other carbon, that is a primary alcohol. • If the alcohol is attached to a carbon that is also attached to one hydrogen and two other carbons, that is a secondary alcohol. • If the alcohol is attached to a carbon ...
... • If the alcohol is attached to a carbon that is also attached to two hydrogens and one other carbon, that is a primary alcohol. • If the alcohol is attached to a carbon that is also attached to one hydrogen and two other carbons, that is a secondary alcohol. • If the alcohol is attached to a carbon ...
Alkene - Synthesis
... you can distill the alkene as it is formed…it will be lower boiling than the alcohol…why? Conc H2SO4 or conc H3PO4 act as both acid catalyst and dehydrating agent. After protonation of the alcohol group, the reaction is E1. ...
... you can distill the alkene as it is formed…it will be lower boiling than the alcohol…why? Conc H2SO4 or conc H3PO4 act as both acid catalyst and dehydrating agent. After protonation of the alcohol group, the reaction is E1. ...
The carbonyl group
... causes the B.P of aldehydes and ketones to be higher than similar molecular weight alkanes and others but lower than alcohols which are held together by H-bonds. Aldehyde < Alcohols > Alkane ...
... causes the B.P of aldehydes and ketones to be higher than similar molecular weight alkanes and others but lower than alcohols which are held together by H-bonds. Aldehyde < Alcohols > Alkane ...
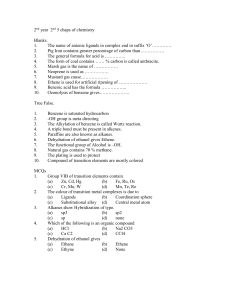
2nd year 2nd 5 chaps of chemistry
... The name of anionic ligands in complex end in suffix ‘O’…………. ...
... The name of anionic ligands in complex end in suffix ‘O’…………. ...
cetomacrogol 1000 ph. eur.
... Cetomacrogol 1000 belongs to the class of polyoxyethylene alkyl ethers, that are nonionic surfactants produced by the polyethoxylation of linear fatty alcohols; they are generally mixtures of polymers of slightly different molecular weights ...
... Cetomacrogol 1000 belongs to the class of polyoxyethylene alkyl ethers, that are nonionic surfactants produced by the polyethoxylation of linear fatty alcohols; they are generally mixtures of polymers of slightly different molecular weights ...
Aldehydes and ketones
... Benedict solution is another reagent that can be used to test for aldehydes. (a) ...
... Benedict solution is another reagent that can be used to test for aldehydes. (a) ...
labPhuc NguyenLab
... the presents of alkene. The precipitation turns into a brownish red color. This process was an E1, because it showed first order kinetics as the breaking of the C-H bond occurred after the rate limiting step. The primary reactant, 4-methylcyclohexanol is a secondary alcohol, and was therefore requir ...
... the presents of alkene. The precipitation turns into a brownish red color. This process was an E1, because it showed first order kinetics as the breaking of the C-H bond occurred after the rate limiting step. The primary reactant, 4-methylcyclohexanol is a secondary alcohol, and was therefore requir ...
TYPES OF HYBRIDIZATION AND GEOMETRY OF MOLECULES
... Choose the chain that include both of them even if this is not the longest chain. The name should include suffixes indicate presence of both the OH group and the unsaturated groups. The OH group takes precedence over the double or triple bonds in getting the lower number. ...
... Choose the chain that include both of them even if this is not the longest chain. The name should include suffixes indicate presence of both the OH group and the unsaturated groups. The OH group takes precedence over the double or triple bonds in getting the lower number. ...
Slide 1
... Classification of Hydrocarbons are based on the type of bonds they form Single Bonds have a maximum number of hydrogen atoms attached to their carbon chains ...
... Classification of Hydrocarbons are based on the type of bonds they form Single Bonds have a maximum number of hydrogen atoms attached to their carbon chains ...
Organic Functional Groups Organic Functional Groups
... • Remember that Fatty acids are long hydrocarbon chains ...
... • Remember that Fatty acids are long hydrocarbon chains ...
Oxidation of alcohols
... Let us look at the basic reaction of an alcohol with a strong oxidising agent. ...
... Let us look at the basic reaction of an alcohol with a strong oxidising agent. ...
Organic Lab
... called ketones. Aldehydes are intermediates in the oxidation of alcohols. For example, when ethyl alcohol is oxidized, the product is first acetaldehyde, which is then further oxidized to acetic acid: ethanolacetaldehydeacetic acid Ketones are the end product of the oxidation of alcohols in which ...
... called ketones. Aldehydes are intermediates in the oxidation of alcohols. For example, when ethyl alcohol is oxidized, the product is first acetaldehyde, which is then further oxidized to acetic acid: ethanolacetaldehydeacetic acid Ketones are the end product of the oxidation of alcohols in which ...
Professor Marina Gatti
... haloalkanes. SN2 and SN1 mechanisms: factors influencing the reaction kinetics. E1 and E2 mechanisms. Competition between substitution and elimination. Alcohols, phenols and ethers. Nomenclature and acid-base properties of alcohols. Reactivity of alcohols connected with breaking of the R-OH bond: re ...
... haloalkanes. SN2 and SN1 mechanisms: factors influencing the reaction kinetics. E1 and E2 mechanisms. Competition between substitution and elimination. Alcohols, phenols and ethers. Nomenclature and acid-base properties of alcohols. Reactivity of alcohols connected with breaking of the R-OH bond: re ...
Esterification and Odors of Esters
... The esters synthesized should have the following scents: isoamyl acetate (banana), ethyl acetate (fruity), methyl salicylate (wintergreen). A collection of other “scented” chemicals can be found with the ester synthesis kit in Dabney 114. ...
... The esters synthesized should have the following scents: isoamyl acetate (banana), ethyl acetate (fruity), methyl salicylate (wintergreen). A collection of other “scented” chemicals can be found with the ester synthesis kit in Dabney 114. ...
Pre DP Chemistry 2 Organic Chemistry
... consists of ONLY carbon and hydrogen. The carbon atoms are bonded together to form the "backbone" to which the hydrogen atoms are ...
... consists of ONLY carbon and hydrogen. The carbon atoms are bonded together to form the "backbone" to which the hydrogen atoms are ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.