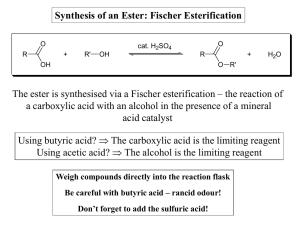
Synthesis of an Ester: Fischer Esterification The ester is synthesised
... Weigh compounds directly into the reaction flask Be careful with butyric acid – rancid odour! Don’t forget to add the sulfuric acid! ...
... Weigh compounds directly into the reaction flask Be careful with butyric acid – rancid odour! Don’t forget to add the sulfuric acid! ...
Chapter 17 Aldehydes and Ketones
... parent alkane to -al. • Because the carbonyl group of an aldehyde can only be at the end of a parent chain and numbering must start with it as carbon-1, there is no need to use a number to locate the aldehyde group. • For unsaturated aldehydes, indicate the presence of a carbon-carbon double bond by ...
... parent alkane to -al. • Because the carbonyl group of an aldehyde can only be at the end of a parent chain and numbering must start with it as carbon-1, there is no need to use a number to locate the aldehyde group. • For unsaturated aldehydes, indicate the presence of a carbon-carbon double bond by ...
Notetakers
... Ethanol is already partially oxidized, so it releases less energy than burning and alkane of comparable mass. However, it can be obtained by the fermentation of biomass; thus, in some countries it is mixed with gasoline to produce “gasohol” which decreases dependence on crude oil. Oxidation of a ...
... Ethanol is already partially oxidized, so it releases less energy than burning and alkane of comparable mass. However, it can be obtained by the fermentation of biomass; thus, in some countries it is mixed with gasoline to produce “gasohol” which decreases dependence on crude oil. Oxidation of a ...
Functional Groups - World of Teaching
... thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching. ...
... thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching. ...
Nuclear Debate - Noadswood Science
... continuous as long as ethene and steam are fed into one end of the reaction vessel, ethanol will be produced These features make it an efficient process, but as ethene is made ...
... continuous as long as ethene and steam are fed into one end of the reaction vessel, ethanol will be produced These features make it an efficient process, but as ethene is made ...
Gas Chromatography: Analyzing Alkene Isomers David L. Flanigan
... Introduction: In this experiment a secondary alcohol (2-methyl-2-butanol) was dehydrated using a dilute solution of sulfuric acid. The reaction resulted in a mixture of alkenes that was purified by distillation and analyzed using gas chromatography. Theoretical Background: Alcohols can be dehydrate ...
... Introduction: In this experiment a secondary alcohol (2-methyl-2-butanol) was dehydrated using a dilute solution of sulfuric acid. The reaction resulted in a mixture of alkenes that was purified by distillation and analyzed using gas chromatography. Theoretical Background: Alcohols can be dehydrate ...
NAT 5 Unit 2 Natures Chem Booklet 2 Everyday
... Some of this biodiesel is made from waste cooking oil and rapeseed oil. Such fuels are carbon neutral, which means that they release only as much carbon dioxide when they burn as was used to make the original oil by photosynthesis. This helps to reduce global warming. However, some people are concer ...
... Some of this biodiesel is made from waste cooking oil and rapeseed oil. Such fuels are carbon neutral, which means that they release only as much carbon dioxide when they burn as was used to make the original oil by photosynthesis. This helps to reduce global warming. However, some people are concer ...
EX. Draw the structure of
... Naming and Drawing Alkyl Halides: Identify the root Identify the prefix: Name and number any alkyl side groups. Insert the number(s) of the carbon atom(s) bonded to the halogen(s). Use the prefix(es) that identify the specific halogen(s) ...
... Naming and Drawing Alkyl Halides: Identify the root Identify the prefix: Name and number any alkyl side groups. Insert the number(s) of the carbon atom(s) bonded to the halogen(s). Use the prefix(es) that identify the specific halogen(s) ...
No Slide Title
... from either side of the carbocation. This gives a mixture of alkenes from unsymmetrical alcohols... ...
... from either side of the carbocation. This gives a mixture of alkenes from unsymmetrical alcohols... ...
+ H 2 O(l) - Knockhardy
... from either side of the carbocation. This gives a mixture of alkenes from unsymmetrical alcohols... ...
... from either side of the carbocation. This gives a mixture of alkenes from unsymmetrical alcohols... ...
Elimination Reactions
... The mechanism of the dehydration of an alcohol to form an alkene is exactly the opposite of the addition of water to an alkene to form an alcohol (microscopic reversibility) Sulfuric acid pulls water away from the reaction and makes the elimination thermodynamically ...
... The mechanism of the dehydration of an alcohol to form an alkene is exactly the opposite of the addition of water to an alkene to form an alcohol (microscopic reversibility) Sulfuric acid pulls water away from the reaction and makes the elimination thermodynamically ...
Period 5
... because it was found to damage the environment •Another compound containing halogens is trichloroethane (C₂H₃Cl₃ ) which, even though it can cause severe health problems, is used in dry-cleaning solutions ...
... because it was found to damage the environment •Another compound containing halogens is trichloroethane (C₂H₃Cl₃ ) which, even though it can cause severe health problems, is used in dry-cleaning solutions ...
Topic 10 IB Chemistry Definitions
... A reaction in which the reactant is added across a C=C bond, converting it to a C-C bond. Addition reactions with water requires an H2SO4 catalyst. Addition reactions with hydrogen use Ni as catalyst. ...
... A reaction in which the reactant is added across a C=C bond, converting it to a C-C bond. Addition reactions with water requires an H2SO4 catalyst. Addition reactions with hydrogen use Ni as catalyst. ...
The term “Chromic Acid” actually refers to a collection of compounds
... contain the chromate ion, CrO42-, and have an intense yellow color. Dichromate salts contain the dichromate ion, Cr2O72−, and have an intense orange color. Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and indust ...
... contain the chromate ion, CrO42-, and have an intense yellow color. Dichromate salts contain the dichromate ion, Cr2O72−, and have an intense orange color. Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and indust ...
102 Lecture Ch17
... (except PCC, which forms aldehydes) • Aldehydes can be oxidized to carboxylic acids with most oxidizing agents, such as Tollens’reagent (AgNO3/NH3) - alcohols do not react with Tollens CrO3 OH ...
... (except PCC, which forms aldehydes) • Aldehydes can be oxidized to carboxylic acids with most oxidizing agents, such as Tollens’reagent (AgNO3/NH3) - alcohols do not react with Tollens CrO3 OH ...
Preparation and Reaction of Carboxylic Acids - IDC
... If E is a strong electrophile, as in the first equation, it will attack the nucleophilic oxygen of the carboxylic acid directly, giving a positively charged intermediate which then loses a proton. If E is a weak electrophile, such as an alkyl halide, it is necessary to convert the carboxylic acid to ...
... If E is a strong electrophile, as in the first equation, it will attack the nucleophilic oxygen of the carboxylic acid directly, giving a positively charged intermediate which then loses a proton. If E is a weak electrophile, such as an alkyl halide, it is necessary to convert the carboxylic acid to ...
15alcpp - Knockhardy
... produced from seeds. It can be used in any diesel engine, either neat or mixed with petroleum diesel. ...
... produced from seeds. It can be used in any diesel engine, either neat or mixed with petroleum diesel. ...
Chapter 23 - Simpson County Schools
... ketones that are water-soluble and easily dissolve in the bloodstream to be transported to tissues. At the same time, some of these ketones are reduced in the liver and the alcohol product is released in to the blood. ...
... ketones that are water-soluble and easily dissolve in the bloodstream to be transported to tissues. At the same time, some of these ketones are reduced in the liver and the alcohol product is released in to the blood. ...
Alcohol

In chemistry, an alcohol is any organic compound in which the hydroxyl functional group (–OH) is bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethyl alcohol (ethanol), the predominant alcohol in alcoholic beverages.The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name. The suffix -ol in non-systematic names (such as paracetamol or cholesterol) also typically indicates that the substance includes a hydroxyl functional group and, so, can be termed an alcohol. But many substances, particularly sugars (examples glucose and sucrose) contain hydroxyl functional groups without using the suffix. An important class of alcohols, of which methanol and ethanol are the simplest members is the saturated straight chain alcohols, the general formula for which is CnH2n+1OH.