Problem Set: Empirical and Molecular Formulas
... 4. Using the same reaction for the production of ammonia as in #3, determine the percent yield when 400.0 kg of H2 are added to an excess of N2, and 1040. kg of NH3 are produced. ...
... 4. Using the same reaction for the production of ammonia as in #3, determine the percent yield when 400.0 kg of H2 are added to an excess of N2, and 1040. kg of NH3 are produced. ...
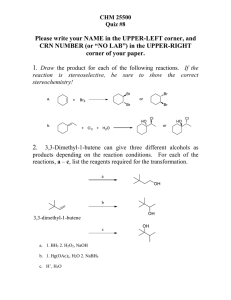
Honors Chemistry Organic Chemistry
... b. carcinogen in burned meat and cigarettes c. organic bases d. hydrogenation e. hydroxyl f. carboxyl g. carbonyl h. containing benzene or benzene-like structures i. from wood distillation / metabolized into formaldehyde j. benzene as a substituent k. reaction in the formation of esters l. phenol ...
... b. carcinogen in burned meat and cigarettes c. organic bases d. hydrogenation e. hydroxyl f. carboxyl g. carbonyl h. containing benzene or benzene-like structures i. from wood distillation / metabolized into formaldehyde j. benzene as a substituent k. reaction in the formation of esters l. phenol ...
Homework #7, Graded Answers
... 29.) Write an equation for each reaction. a.) 1-butanol is heated to 140°C in the presence of sulfuric acid H2SO4 OH ...
... 29.) Write an equation for each reaction. a.) 1-butanol is heated to 140°C in the presence of sulfuric acid H2SO4 OH ...
18 Important and sometimes forgotten) organic transformations
... •Reagents used are iodine and silver acetate in wet acetic acid ...
... •Reagents used are iodine and silver acetate in wet acetic acid ...
3.8 ADDITION OF WATER TO AN ALKENE H or enzyme + H-O
... by a suitable method, either by appropriate radiation, or a free radical initiator such as a peroxide molecule (the most common way of producing them industrially). By one means or another, we produce free radical ethylene molecules which can pair up with each other to form a long chain: ...
... by a suitable method, either by appropriate radiation, or a free radical initiator such as a peroxide molecule (the most common way of producing them industrially). By one means or another, we produce free radical ethylene molecules which can pair up with each other to form a long chain: ...
Document
... In this rearrangement, an electron is donated through space; the other electron to form the bond comes from a bond to this atom. The initial cleavage does not cause fragmentation, only rearrangement and transfer of the radical site. This new radical site then initiates an alpha cleavage, resulting i ...
... In this rearrangement, an electron is donated through space; the other electron to form the bond comes from a bond to this atom. The initial cleavage does not cause fragmentation, only rearrangement and transfer of the radical site. This new radical site then initiates an alpha cleavage, resulting i ...
What is an addition reaction
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
... In a condensation reaction, two organic molecules react together to produce one larger organic molecule and a molecule of water. For this type of reaction to occur, one of the molecules must have a hydroxyl group, and the other must have an active site with hydrogens, such as another hydroxyl group, ...
CHAPTER-6 DEHYDROHALOGENATION OF ALKYL HALIDES
... – Strong bases such as alkoxides favor elimination ...
... – Strong bases such as alkoxides favor elimination ...
Types of Reactions in Organic Chemistry Chemistry
... Types of Reactions in Organic Chemistry The mechanism of a reaction is the detailed step-by-step description of how the overall reaction occurs. A substitution reaction is a chemical reaction in which an atom or group of atoms in a molecule is replaced by another atom or group of atoms. A substitut ...
... Types of Reactions in Organic Chemistry The mechanism of a reaction is the detailed step-by-step description of how the overall reaction occurs. A substitution reaction is a chemical reaction in which an atom or group of atoms in a molecule is replaced by another atom or group of atoms. A substitut ...
AMINO ACIDS Ethan Secor, John N. Gitua (Mentor)
... mixture. The reaction mixture was gradually warmed to room temperature. Carbon dioxide was introduced from the sublimation of dry ice. Finally, hydrochloric acid was added to quench the reaction. The organic layer was extracted, and the aqueous layer was neutralized with sodium hydroxide. Diethyl et ...
... mixture. The reaction mixture was gradually warmed to room temperature. Carbon dioxide was introduced from the sublimation of dry ice. Finally, hydrochloric acid was added to quench the reaction. The organic layer was extracted, and the aqueous layer was neutralized with sodium hydroxide. Diethyl et ...
TYPES OF REACTIONS IN ORGANIC CHEMISTRY
... A chlorine atom attacks the methane molecule to form hydrogen chloride and a methyl free radical. ...
... A chlorine atom attacks the methane molecule to form hydrogen chloride and a methyl free radical. ...
Enantiospecific skeleton expanding cross
... pathway for the modification and expansion of α-hydroxy carbonyls, which are themselves readily available from the chiral pool. Grignard reagents possessing a range of potential substituents are reacted under mild conditions with a triflate modified carbonyl at the α-carbon to bind a wide range of s ...
... pathway for the modification and expansion of α-hydroxy carbonyls, which are themselves readily available from the chiral pool. Grignard reagents possessing a range of potential substituents are reacted under mild conditions with a triflate modified carbonyl at the α-carbon to bind a wide range of s ...
8th Grade - Chemistry
... A chart of the elements showing the repeating patterns of their properties. The elements in a group, or family, have similar properties, and the properties such as the number of valence electrons can be predicted from their location. The reactivity of metals decreases from left to right across the p ...
... A chart of the elements showing the repeating patterns of their properties. The elements in a group, or family, have similar properties, and the properties such as the number of valence electrons can be predicted from their location. The reactivity of metals decreases from left to right across the p ...
Combustion, Addition and Elimination Objective Combustion Example
... is weaker than the single bond, this bond can break and then we have free bonding electrons where another element or functional group can be added. In general: ...
... is weaker than the single bond, this bond can break and then we have free bonding electrons where another element or functional group can be added. In general: ...
Year 9 Homework Task 9E-5 Reactions 5-7
... Task: Complete the diagrams. Describe, in words, what happens at each stage of the reaction. Explain what happens during the reaction, using particle diagrams. ...
... Task: Complete the diagrams. Describe, in words, what happens at each stage of the reaction. Explain what happens during the reaction, using particle diagrams. ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... making the carbon electrophilic Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce ...
... making the carbon electrophilic Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce ...
ch02-chemistry
... 2. _______ Two hydrogen atoms share valence electrons to form a hydrogen molecule 3. _______ A weak attraction between a slightly positive hydrogen atom in one molecule and a slightly negative oxygen or nitrogen atom in another 4. _______ The attraction of a cation to an anion ...
... 2. _______ Two hydrogen atoms share valence electrons to form a hydrogen molecule 3. _______ A weak attraction between a slightly positive hydrogen atom in one molecule and a slightly negative oxygen or nitrogen atom in another 4. _______ The attraction of a cation to an anion ...
Assignment 2 Group A and B
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
... 9) Which of the following alcohols can be prepared by the reaction of methyl formate with excess Grignard reagent? A) 1-pentanol B) 2-pentanol C) 3-pentanol D) 2-methyl-2-pentanol E) 3-methyl-3-pentanol 10) What reagent(s) would you use to accomplish the following conversion? ...
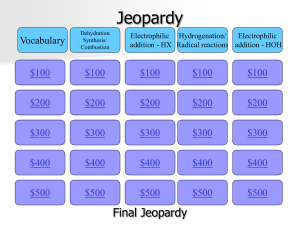
Jeopardy
... formation of 2-methyl-2-bromopentane from the electrophilic addition reaction between 2methylpent-2-ene and HBr. Make certain to include the rate determining step and use ...
... formation of 2-methyl-2-bromopentane from the electrophilic addition reaction between 2methylpent-2-ene and HBr. Make certain to include the rate determining step and use ...
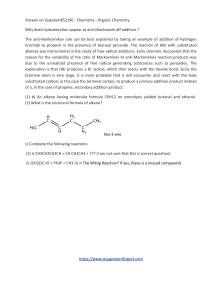
Answer on Question#52196 - Chemistry
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
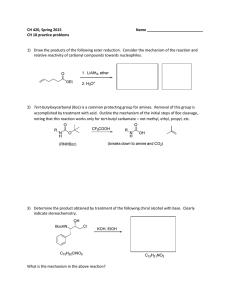
CH 420, Spring 2015 Name ___________________________ CH 18 practice problems
... noting that this reaction works only for tert-butyl carbamate – not methyl, ethyl, propyl, etc. ...
... noting that this reaction works only for tert-butyl carbamate – not methyl, ethyl, propyl, etc. ...
$doc.title
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
... http://www.chem.wisc.edu/areas/clc (Resource page) Reactions of Alcohols #8: Reaction of a 1° Alcohol with Hydrogen Halides ...
Hofmann–Löffler reaction

The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.