C h e m g u id e –... ACID ANHYDRIDES: REACTIONS WITH WATER, ALCOHOLS AND PHENOLS
... produced. (ii) Ethanoic acid is formed instead of hydrogen chloride gas as the second product of the reaction. O ...
... produced. (ii) Ethanoic acid is formed instead of hydrogen chloride gas as the second product of the reaction. O ...
chapter 4 review_package
... iv. How many moles of Si4H10 are needed to just react with 1.204 x 1026 molecules of oxygen? ...
... iv. How many moles of Si4H10 are needed to just react with 1.204 x 1026 molecules of oxygen? ...
Here is the Original File - University of New Hampshire
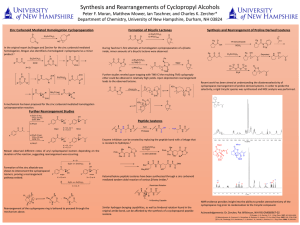
... imide, minor amounts of a bicyclic lactone were observed. ...
... imide, minor amounts of a bicyclic lactone were observed. ...
ZADANIE 1
... 6.7), 4.40 (q, 1 H, J = 6.7), 12.0 (s, 1 H; this signal may be broadened and shifted upfield due to minimal amounts of water); 13C NMR (CDCl3) δ: 20.9, 52.0, 176.0. 3. Discussion The present procedure is based on the method published by Fu et al.5 The yields are increased by the very slow addition o ...
... 6.7), 4.40 (q, 1 H, J = 6.7), 12.0 (s, 1 H; this signal may be broadened and shifted upfield due to minimal amounts of water); 13C NMR (CDCl3) δ: 20.9, 52.0, 176.0. 3. Discussion The present procedure is based on the method published by Fu et al.5 The yields are increased by the very slow addition o ...
Final-01 - Yale Department of Chemistry
... we are not only dealing with two cyclohexenes but also the position of the double bond in the ring. A and D are defined. B and C are formed by bromine radical addition to give a tertiary radical that abstracts a hydrogen atom from HBr. In both B and C the reacting conformation must have bromine axia ...
... we are not only dealing with two cyclohexenes but also the position of the double bond in the ring. A and D are defined. B and C are formed by bromine radical addition to give a tertiary radical that abstracts a hydrogen atom from HBr. In both B and C the reacting conformation must have bromine axia ...
5.2. Related mechanisms of halogen chemistry A large variety of
... related to carbonyls or other oxygen containing functional groups. The NOX-free formed SOA in this study is suspected to not contain any different chemical element than carbon, hydrogen and oxygen. Furthermore, in the oxidizing environment of the smog-chamber atmosphere, no chemical reduction will o ...
... related to carbonyls or other oxygen containing functional groups. The NOX-free formed SOA in this study is suspected to not contain any different chemical element than carbon, hydrogen and oxygen. Furthermore, in the oxidizing environment of the smog-chamber atmosphere, no chemical reduction will o ...
Functional Groups
... Properties of Alcohols Compared to alkenes alcohols have higher boiling points. This is explained by the OH group which allows hydrogen bonding. This functional group also makes alcohols polar which allows simple (small) alcohols to dissolve in water. Long chain alcohols act as nonpolar molecules ma ...
... Properties of Alcohols Compared to alkenes alcohols have higher boiling points. This is explained by the OH group which allows hydrogen bonding. This functional group also makes alcohols polar which allows simple (small) alcohols to dissolve in water. Long chain alcohols act as nonpolar molecules ma ...
alcohols, alkyl halides, and nucleophilic substitutions
... 1. Arrange the alcohols tested in order of decreasing experimental reactivity toward Lucas reagent. Consider the aliphatic alcohols first and then consider the aromatic compounds separately. What structural change correlates with reactivity? Assuming this to be an SN1 reaction (see scheme in part B ...
... 1. Arrange the alcohols tested in order of decreasing experimental reactivity toward Lucas reagent. Consider the aliphatic alcohols first and then consider the aromatic compounds separately. What structural change correlates with reactivity? Assuming this to be an SN1 reaction (see scheme in part B ...
Alicyclic esters of phosphoric acids
... The desired product may be obtained from the reaction the bicycloheptadiene may be employed. It is preferable mixture by methods known in the art. For example, the to use a small excess-perhaps from about 5% to about desired product may be obtained by distillation of the 100%—~of the bicycloheptadie ...
... The desired product may be obtained from the reaction the bicycloheptadiene may be employed. It is preferable mixture by methods known in the art. For example, the to use a small excess-perhaps from about 5% to about desired product may be obtained by distillation of the 100%—~of the bicycloheptadie ...
Organic Chemistry
... The organic halogen compound: aryl, heterocyclic, benzylic, and vinylic iodides, chlorides, bromides, and triflates (CF3SO2O-). • alkyl halides with an easily eliminated b hydrogen are rarely used because they undergo b-elimination to give alkenes. • OH groups and the C=O groups of aldehydes, ketone ...
... The organic halogen compound: aryl, heterocyclic, benzylic, and vinylic iodides, chlorides, bromides, and triflates (CF3SO2O-). • alkyl halides with an easily eliminated b hydrogen are rarely used because they undergo b-elimination to give alkenes. • OH groups and the C=O groups of aldehydes, ketone ...
NaBH4 Reduction of Vanillin
... Aside from a lack of selectivity, LAH reacts violently with water and other hydroxylic compounds, and reductions using this reagent must be carried out under non‐protic, anhydrous conditions. This not only limits the solvents with which LAH can be used, but it presents greater challenges in th ...
... Aside from a lack of selectivity, LAH reacts violently with water and other hydroxylic compounds, and reductions using this reagent must be carried out under non‐protic, anhydrous conditions. This not only limits the solvents with which LAH can be used, but it presents greater challenges in th ...
10. Alcohols - The Student Room
... At lower temperatures the reaction is too slow. At higher temperatures the yeast dies and the enzymes denature. Fermentation is done in an absence of air because the presence of air can cause extra reactions to occur. It oxidises the ethanol produced to ethanoic acid (vinegar). Advantages •sugar is ...
... At lower temperatures the reaction is too slow. At higher temperatures the yeast dies and the enzymes denature. Fermentation is done in an absence of air because the presence of air can cause extra reactions to occur. It oxidises the ethanol produced to ethanoic acid (vinegar). Advantages •sugar is ...
Exam 3 - Chemistry
... urea cycle for ridding an organism of ammonia is the conversion of arginosuccinate to the amino acid arginine plus fumarate. a. What reaction is occurring here? ___E2___ b. On the structure ...
... urea cycle for ridding an organism of ammonia is the conversion of arginosuccinate to the amino acid arginine plus fumarate. a. What reaction is occurring here? ___E2___ b. On the structure ...
aldehyde,ketones and Haloalkanes
... Q.12 Give a chemical test to distinguish between the following pairs of compounds :[4] (i) Chlorobenzene and cyclohexylchloride. (ii) Vinyl chloride and Ethyl chloride. (iii) n-Propylbromide and Isopropylbromide. (iv)bromo butane & chloro butane. Q.13 How will you bring about the following conversio ...
... Q.12 Give a chemical test to distinguish between the following pairs of compounds :[4] (i) Chlorobenzene and cyclohexylchloride. (ii) Vinyl chloride and Ethyl chloride. (iii) n-Propylbromide and Isopropylbromide. (iv)bromo butane & chloro butane. Q.13 How will you bring about the following conversio ...
CH 12-3 Power Point
... •Important multi-step synthesis for making new C-C bonds, and 1o, 2o, and 3o alcohols. ...
... •Important multi-step synthesis for making new C-C bonds, and 1o, 2o, and 3o alcohols. ...
ALKENES and SULPHURIC ACID
... This is typical of the reaction with unsymmetrical alkenes. An unsymmetrical alkene has different groups at either end of the carbon-carbon double bond. If sulphuric acid adds to an unsymmetrical alkene like propene, there are two possible ways it could add. You could end up with one of two product ...
... This is typical of the reaction with unsymmetrical alkenes. An unsymmetrical alkene has different groups at either end of the carbon-carbon double bond. If sulphuric acid adds to an unsymmetrical alkene like propene, there are two possible ways it could add. You could end up with one of two product ...
슬라이드 1
... Heck Reaction: Aryl and alkenyl halides react with alkenes in the presence of catalytic amounts of palladium to give net substitution of the halide by the alkenyl group. The reaction is quite general and has been observed for simple alkenes, aryl sustituted alkenes, and electrophilic alkenes such as ...
... Heck Reaction: Aryl and alkenyl halides react with alkenes in the presence of catalytic amounts of palladium to give net substitution of the halide by the alkenyl group. The reaction is quite general and has been observed for simple alkenes, aryl sustituted alkenes, and electrophilic alkenes such as ...
CH 10
... connected to a carbon atom in a structure is replaced by oxygen, nitrogen, or halogen – Not defined as loss of electrons by an atom as in inorganic chemistry ...
... connected to a carbon atom in a structure is replaced by oxygen, nitrogen, or halogen – Not defined as loss of electrons by an atom as in inorganic chemistry ...
Heck Reactions
... total synthesis of (-)-triptolide, (-)-triptonide, (+)triptophenolide, and (+)-triptquinonide." J. Org. Chem. 2000, 65, 2208-2217. An application of Crisp’s method for the synthesis of γ-lactones from β-keto esters. ...
... total synthesis of (-)-triptolide, (-)-triptonide, (+)triptophenolide, and (+)-triptquinonide." J. Org. Chem. 2000, 65, 2208-2217. An application of Crisp’s method for the synthesis of γ-lactones from β-keto esters. ...
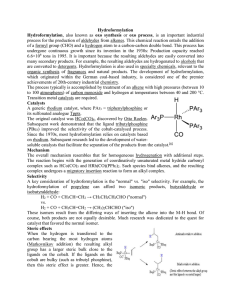
Hydroformylation Hydroformylation, also known as oxo synthesis or
... 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic s ...
... 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic s ...
The Formation of 2,2,4-Trimethyl-2,3-dihydro-1H-1,5
... Reactions of o-phenylenediamine with a dicarboxylic acid can produce several different products depending on the specific conditions [1]. In the presence of cyclization agents such as hydrochloric acid or polyphosphoric acid, these reactions have been reported to give benzimidazoles [2,3]. This is a ...
... Reactions of o-phenylenediamine with a dicarboxylic acid can produce several different products depending on the specific conditions [1]. In the presence of cyclization agents such as hydrochloric acid or polyphosphoric acid, these reactions have been reported to give benzimidazoles [2,3]. This is a ...
AP CHEMISTRY PROBLEMS ENTHALPY, ENTROPY, AND FREE
... 13. For the reaction 2NO2 (g) ⇆N2O4 (g) , the values of ΔH° and ΔS° are -58.03 kJ and -176.6 J/K respectively. What is the value of ΔG° at 298 K? Assuming that ΔH° and ΔS° do not depend on temperature, at what temperature is ΔG°=O? Is ΔG° negative above or below this ...
... 13. For the reaction 2NO2 (g) ⇆N2O4 (g) , the values of ΔH° and ΔS° are -58.03 kJ and -176.6 J/K respectively. What is the value of ΔG° at 298 K? Assuming that ΔH° and ΔS° do not depend on temperature, at what temperature is ΔG°=O? Is ΔG° negative above or below this ...
CBSE Guess Papers Chemistry Class XII(12th) 2006
... 23.a) What are essential and non- essential amino acids? Give two examples of each. b) Give reasons for the following: i) Amino acids have relatively higher melting point as compared to corresponding halo acids. ii) Amino acids are amphoteric in nature. iii) On electrolysis in acidic solution amino ...
... 23.a) What are essential and non- essential amino acids? Give two examples of each. b) Give reasons for the following: i) Amino acids have relatively higher melting point as compared to corresponding halo acids. ii) Amino acids are amphoteric in nature. iii) On electrolysis in acidic solution amino ...
Hofmann–Löffler reaction

The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.