(Z)-Tamoxifen and Tetrasubstituted Alkenes and Dienes via a Regio
... π-orbitals in the addition component. Thus the poor result with the methylmagnesium chloride (entry 8) is due to the inefficiency of the carbometalation and not to the subsequent cross-coupling. The yield of the Grignard additions to propargyl alcohols can be enhanced, in some cases, by the addition ...
... π-orbitals in the addition component. Thus the poor result with the methylmagnesium chloride (entry 8) is due to the inefficiency of the carbometalation and not to the subsequent cross-coupling. The yield of the Grignard additions to propargyl alcohols can be enhanced, in some cases, by the addition ...
Electrophilic Additions: Alkenes Addition of Hydrogen Halides
... Endergonic reaction: late transition state resembles products (II). ...
... Endergonic reaction: late transition state resembles products (II). ...
Copper perchlorate: Efficient acetylation catalyst
... achieved in contrast to some other catalysts. Acylal formation from aldehydes is found to be very rapid and at the same time keto groups remain unchanged. Thus the transition metal perclorates are better for acetylation hederoatoms and aldehydes than other metal perclorates and metal triflates. It m ...
... achieved in contrast to some other catalysts. Acylal formation from aldehydes is found to be very rapid and at the same time keto groups remain unchanged. Thus the transition metal perclorates are better for acetylation hederoatoms and aldehydes than other metal perclorates and metal triflates. It m ...
Aromatic Compounds
... An acyl group, -COR, is substituted onto an aromatic ring • The reactive electrophile is a resonance-stabilized acyl cation • An acyl cation is stabilized by interaction of the vacant orbital on carbon with lone-pair electrons on the neighboring oxygen • Because of stabilization, no carbocation rear ...
... An acyl group, -COR, is substituted onto an aromatic ring • The reactive electrophile is a resonance-stabilized acyl cation • An acyl cation is stabilized by interaction of the vacant orbital on carbon with lone-pair electrons on the neighboring oxygen • Because of stabilization, no carbocation rear ...
Studies toward the Stereoselective Synthesis of the
... Mycotoxins are toxic secondary metabolites produced by fungi and they are the causative agents of various diseases in man and his domestic animals. Human beings and animals get the diseases, commonly called mycotoxicoses through the ingestion of foods or feeds contaminated by these toxic fungal meta ...
... Mycotoxins are toxic secondary metabolites produced by fungi and they are the causative agents of various diseases in man and his domestic animals. Human beings and animals get the diseases, commonly called mycotoxicoses through the ingestion of foods or feeds contaminated by these toxic fungal meta ...
Topic 8 notes - A
... A substitution reaction is one in which one atom or group of atoms on the organic molecule is directly replaced by another. In this reaction the H atom on the alkane is replaced by a Cl atom. Since all the carbon atoms in alkanes are attached to four other atoms, it is not possible to add another sp ...
... A substitution reaction is one in which one atom or group of atoms on the organic molecule is directly replaced by another. In this reaction the H atom on the alkane is replaced by a Cl atom. Since all the carbon atoms in alkanes are attached to four other atoms, it is not possible to add another sp ...
english,
... The liberated molecule of glucose has also the possibility of reacting in a secondary condensation reaction, though transglucosylation is preferred. Unfortunately, a side reaction of liberated glucose and cellobiose is also possible. TLC was used to monitor the increase of product concentration. Aft ...
... The liberated molecule of glucose has also the possibility of reacting in a secondary condensation reaction, though transglucosylation is preferred. Unfortunately, a side reaction of liberated glucose and cellobiose is also possible. TLC was used to monitor the increase of product concentration. Aft ...
07. Aldehydes and ketones
... The chemical consequences of this bond polarity will be are become apparent during our discussions of the reactions of carbonyl groups. We shall find that the positive carbon can react with bases and that much of the chemistry оf the carbonyl function corresponds to that of а relatively stable carb ...
... The chemical consequences of this bond polarity will be are become apparent during our discussions of the reactions of carbonyl groups. We shall find that the positive carbon can react with bases and that much of the chemistry оf the carbonyl function corresponds to that of а relatively stable carb ...
Document
... Many are chiral and occur in single enantiomer form. Some of these compounds are pharmacologically important, e. g., quinine and atropine. Others have dangerous natures, e. g., morphine and strychnine. Some alkaloids have been used to separate the enantiomers of chiral carboxylic acids. This resolut ...
... Many are chiral and occur in single enantiomer form. Some of these compounds are pharmacologically important, e. g., quinine and atropine. Others have dangerous natures, e. g., morphine and strychnine. Some alkaloids have been used to separate the enantiomers of chiral carboxylic acids. This resolut ...
View/Open - AURA - Alfred University
... triflic acid, either already present or generated in situ, were responsible for reaction progress. To test this, a reaction of naphthalenemethanol (38), 3,4-dihydro-2H-pyran, and PEDOT in benzene was performed as well as a similar reaction using Amberlite, a known polymerbound acid, in the place of ...
... triflic acid, either already present or generated in situ, were responsible for reaction progress. To test this, a reaction of naphthalenemethanol (38), 3,4-dihydro-2H-pyran, and PEDOT in benzene was performed as well as a similar reaction using Amberlite, a known polymerbound acid, in the place of ...
A Mild and Convenient Conversion of Ketones to the Corresponding
... compared to the large excesses required in the other procedures), (b) mild reaction conditions (room temperature, neutral pH, and the use of common aprotic solvents), (c) the inertness of most functional groups toward catecholborane (only aldehydes are reduced faster than tosylhydrazones), and (d) f ...
... compared to the large excesses required in the other procedures), (b) mild reaction conditions (room temperature, neutral pH, and the use of common aprotic solvents), (c) the inertness of most functional groups toward catecholborane (only aldehydes are reduced faster than tosylhydrazones), and (d) f ...
hydroxy- and oxoacids. heterofunctional compounds of benzene
... interconversion between two forms This phenomenon is called tautomerism. Forms which turn one into another are tautomers and their mutual transition are tautomeric transformations. If tautomers are substances with carbonyl and enol groups (for example, isomerism of acetoacetic ester), than tautomeri ...
... interconversion between two forms This phenomenon is called tautomerism. Forms which turn one into another are tautomers and their mutual transition are tautomeric transformations. If tautomers are substances with carbonyl and enol groups (for example, isomerism of acetoacetic ester), than tautomeri ...
No Slide Title
... Deborah T. Hung, Jennie B. Nerenberg, and Stuart L. Schreiber* Contribution from the Howard Hughes Medical Institute, Department of Chemistry and Chemical ...
... Deborah T. Hung, Jennie B. Nerenberg, and Stuart L. Schreiber* Contribution from the Howard Hughes Medical Institute, Department of Chemistry and Chemical ...
Lecture - Ch 24
... – 1H NMR spectrum shows a 9-proton singlet, a one-proton quartet, and a 3-proton doublet – Absorption due to the amine protons is not ...
... – 1H NMR spectrum shows a 9-proton singlet, a one-proton quartet, and a 3-proton doublet – Absorption due to the amine protons is not ...
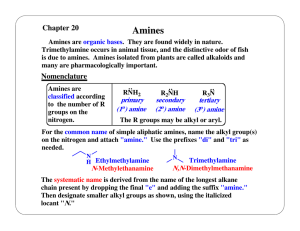
Lecture 10 Carbon-Nitrogen Bonds Formation I
... 4.1 Principles The methods for the formation of bonds between nitrogen and aliphatic carbon can be broadly divided into two categories: (i) reaction of nucleophilic nitrogen with electrophilic carbon, and (ii) reaction of electrophilic nitrogen with nucleophilic carbon. In this section, we will try ...
... 4.1 Principles The methods for the formation of bonds between nitrogen and aliphatic carbon can be broadly divided into two categories: (i) reaction of nucleophilic nitrogen with electrophilic carbon, and (ii) reaction of electrophilic nitrogen with nucleophilic carbon. In this section, we will try ...
The Acid Hydrolysis Mechanism of Acetals Catalyzed
... Figure 2. (a) 1H NMR spectrum of 1 with 75 equivalents of 2. (b) 1H NMR spectrum after addition of one equivalent of NEt4+. (c) 1H NMR spectrum 1 in H2O shown for comparison. All spectra were recorded at pH 10.0, 100 mM K2CO3, 22 °C, in H2O with equal concentrations of 2 using Watergate solvent supp ...
... Figure 2. (a) 1H NMR spectrum of 1 with 75 equivalents of 2. (b) 1H NMR spectrum after addition of one equivalent of NEt4+. (c) 1H NMR spectrum 1 in H2O shown for comparison. All spectra were recorded at pH 10.0, 100 mM K2CO3, 22 °C, in H2O with equal concentrations of 2 using Watergate solvent supp ...
Learning Guide for Chapter 9 - Alkyl Halides I
... high polarity solvent - to dissolve charged reagents aprotic solvent - hydrogen bonding slows down the reaction surrounds Nu/base, makes it hard to react What kind of solvent do SN1 reactions require? polarity isn't important - reagents are not charged, dissolve easily protic solvent - stabilizes th ...
... high polarity solvent - to dissolve charged reagents aprotic solvent - hydrogen bonding slows down the reaction surrounds Nu/base, makes it hard to react What kind of solvent do SN1 reactions require? polarity isn't important - reagents are not charged, dissolve easily protic solvent - stabilizes th ...
Orbitals
... proceeding through a nucleophilic addition of a hydride ion (:H–) to the C=O carbon • LiAlH4 and NaBH4 act as if they are donors of hydride ion ...
... proceeding through a nucleophilic addition of a hydride ion (:H–) to the C=O carbon • LiAlH4 and NaBH4 act as if they are donors of hydride ion ...
Rapid and Efficient Functionalized Ionic Liquid-Catalyzed
... catalytic activity was obtained in acetate based IL among 9 TSILs [32]. Subsequently, they synthesized a new IL, (2-hydroxyethyl)-trimethyl-ammonium (S)-2-pyrrolidinecarboxylic acid ([Choline][Pro]) from biorenewable and nontoxic raw materials, which has shown to be an efficient catalyst for the dir ...
... catalytic activity was obtained in acetate based IL among 9 TSILs [32]. Subsequently, they synthesized a new IL, (2-hydroxyethyl)-trimethyl-ammonium (S)-2-pyrrolidinecarboxylic acid ([Choline][Pro]) from biorenewable and nontoxic raw materials, which has shown to be an efficient catalyst for the dir ...
molecules Palladium and Organocatalysis: An Excellent Recipe for Asymmetric Synthesis
... corresponding chiral enolates are protonated with an achiral proton source, such as Meldrum’s acid [41]. As depicted in Scheme 9, a wide range of tertiary-substituted cyclic ketones can be prepared in very good yields and enantioselectivities using the chiral phosphinooxazoline (PHOX) as ligand for ...
... corresponding chiral enolates are protonated with an achiral proton source, such as Meldrum’s acid [41]. As depicted in Scheme 9, a wide range of tertiary-substituted cyclic ketones can be prepared in very good yields and enantioselectivities using the chiral phosphinooxazoline (PHOX) as ligand for ...
CHEM 494 Lecture 10b - UIC Department of Chemistry
... Meissner 1819: To me it seems wholly appropriate to refer to those plant substances currently known by the names of alkalis, but alkaloids, since some of their properties they differ from alkalis considerably, and would thus find their place before the plant acids in the field of plant chemistry. Ja ...
... Meissner 1819: To me it seems wholly appropriate to refer to those plant substances currently known by the names of alkalis, but alkaloids, since some of their properties they differ from alkalis considerably, and would thus find their place before the plant acids in the field of plant chemistry. Ja ...
C - Deans Community High School
... a hint!!!! This is a primary alcohol because the OH group is attached to a carbon which is bonded to only one other carbon. H ...
... a hint!!!! This is a primary alcohol because the OH group is attached to a carbon which is bonded to only one other carbon. H ...
Mechanistic notation
... The more stable the carbocation, the faster it is formed. Tertiary carbocations are more stable than secondary, which are more stable than primary, which are more stable than methyl. Tertiary alcohols react faster than secondary, which react faster than primary, which react faster than methanol. ...
... The more stable the carbocation, the faster it is formed. Tertiary carbocations are more stable than secondary, which are more stable than primary, which are more stable than methyl. Tertiary alcohols react faster than secondary, which react faster than primary, which react faster than methanol. ...
Characterization of Amide Bond Conformers for a Novel
... attributed to imine hydrogen of conformer ap in DMSO-d6 and in CDCl3 [7]. Furthermore, in the ap conformation, the two dipolar moments (Cδ+=Oδ− and Nδ−-Hδ+) are aligned, which does not occur in the sp conformer [21]. According to literature reports, the presence of conformers for the NAH compounds i ...
... attributed to imine hydrogen of conformer ap in DMSO-d6 and in CDCl3 [7]. Furthermore, in the ap conformation, the two dipolar moments (Cδ+=Oδ− and Nδ−-Hδ+) are aligned, which does not occur in the sp conformer [21]. According to literature reports, the presence of conformers for the NAH compounds i ...
Hofmann–Löffler reaction

The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.








![ch15[1].](http://s1.studyres.com/store/data/008194241_2-0a33cfb98ac502873dac865380b726e0-300x300.png)