Preparation and Reaction of Carboxylic Acids - IDC
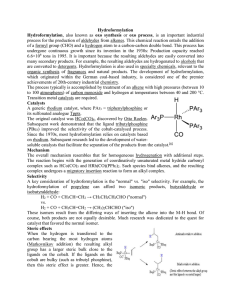
... proceed by way of tetrahedral intermediates (such as A and B in the mechanism diagram) and are common in acyl substitution reactions. Acid catalysis is necessary to increase the electrophilic character of the carboxyl carbon atom, so it will bond more rapidly to the ...
... proceed by way of tetrahedral intermediates (such as A and B in the mechanism diagram) and are common in acyl substitution reactions. Acid catalysis is necessary to increase the electrophilic character of the carboxyl carbon atom, so it will bond more rapidly to the ...
Practice Final Exam, Chemistry 2220, Organic Chem II 1. Rank the
... HBr, then NaOH/heat, then H3O+, then PCC Br2/light, then EtONa, then BH3, then H2O2/NaOH, then PCC Br2/FeBr3, then NaOH, then BH3, then H2O2/NaOH, then CrO3/H2SO4 NBS/heat, then (CH3)3CONa, then H3O+/heat, then PCC ...
... HBr, then NaOH/heat, then H3O+, then PCC Br2/light, then EtONa, then BH3, then H2O2/NaOH, then PCC Br2/FeBr3, then NaOH, then BH3, then H2O2/NaOH, then CrO3/H2SO4 NBS/heat, then (CH3)3CONa, then H3O+/heat, then PCC ...
Selectivity of sodium borohydride
... and is more selective in its reactivity and reduces fewer functional groups. However, both reagents are similar in that all four of the hydrogen atoms present can at least in principle, function as nucleophiles. The selectivity of sodium borohydride towards carbonyl containing compounds such as alde ...
... and is more selective in its reactivity and reduces fewer functional groups. However, both reagents are similar in that all four of the hydrogen atoms present can at least in principle, function as nucleophiles. The selectivity of sodium borohydride towards carbonyl containing compounds such as alde ...
Chemistry - Target Publications
... Q.6. Answer any SIX of the following: i. Identify ‘A’ and ‘B’ in the following reaction : HBr alc.KOH CH3 − CH = CH2 → ‘A’ → ‘B’ ii. Distinguish between lanthanoids and actinoids. iii. How are the following compounds prepared? a. Benzyl alcohol from benzyl chloride. ...
... Q.6. Answer any SIX of the following: i. Identify ‘A’ and ‘B’ in the following reaction : HBr alc.KOH CH3 − CH = CH2 → ‘A’ → ‘B’ ii. Distinguish between lanthanoids and actinoids. iii. How are the following compounds prepared? a. Benzyl alcohol from benzyl chloride. ...
Unit 2 Content Statements
... The stability of the benzene ring is due to the delocalisation of electrons. A benzene ring in which one hydrogen atom has been substituted by another group is known as the phenyl group. The phenyl group has the formula -C6H5. ...
... The stability of the benzene ring is due to the delocalisation of electrons. A benzene ring in which one hydrogen atom has been substituted by another group is known as the phenyl group. The phenyl group has the formula -C6H5. ...
Reductive etherification of substituted cyclohexanones with
... remarkably shape selective reductive etherification of cyclohexanones with bulky substituents at the 4-position, employing secondary alcohols as reductants. Zeolite MCM-22 has recently been introduced as a unique biporous zeolite.1 It has been shown that it contains two independent pore systems,2,3 ...
... remarkably shape selective reductive etherification of cyclohexanones with bulky substituents at the 4-position, employing secondary alcohols as reductants. Zeolite MCM-22 has recently been introduced as a unique biporous zeolite.1 It has been shown that it contains two independent pore systems,2,3 ...
2B Synthesis Organic synthesis of aliphatic and aromatic
... One isomer rotates it in one direction and the other in the opposite direction. ...
... One isomer rotates it in one direction and the other in the opposite direction. ...
Information Regarding Prof
... dihydroxylation includes the C2, C3 hydroxyl groups, protects the hydroxyl group at C2 and uses a tert. butyl ester to withstand the attack of Grignard and enolate reagents. 4) InCl3-Catalyzed direct aldol reactions of glyoxylic acid monohydrate and glyoxylates with various ketones: scope and limita ...
... dihydroxylation includes the C2, C3 hydroxyl groups, protects the hydroxyl group at C2 and uses a tert. butyl ester to withstand the attack of Grignard and enolate reagents. 4) InCl3-Catalyzed direct aldol reactions of glyoxylic acid monohydrate and glyoxylates with various ketones: scope and limita ...
Title Carbonyl reduction with CaH2 and R3SiCl catalyzed by ZnCl2
... 1. N. Greeves, In Comprehensive Organic Synthesis; B. M. Trost and I. Fleming, Eds.; Pergamon Press: Oxford, 1991, vol. 9, p. 1. J. Seyden-Penne, Reductions by the Alumino- and Borohydrides in Organic Synthesis; VHC: 1991. 2. Ohkuma, T.; Hashiguchi, S.; Noyori, R. J. Org. Chem. 1994, ...
... 1. N. Greeves, In Comprehensive Organic Synthesis; B. M. Trost and I. Fleming, Eds.; Pergamon Press: Oxford, 1991, vol. 9, p. 1. J. Seyden-Penne, Reductions by the Alumino- and Borohydrides in Organic Synthesis; VHC: 1991. 2. Ohkuma, T.; Hashiguchi, S.; Noyori, R. J. Org. Chem. 1994, ...
C3 Knowledge Test – Higher Tier 1. Why was Mendeleev`s periodic
... Describe what happens to bonds in exothermic and endothermic reactions. Describe how a catalyst works, in terms of energy. Give an advantage and a disadvantage of hydrogen fuel cells. What colour flame will the following ions burn with; K+, Na+, Ca2+, Ba2+, Li+? What colour precipitate is forme ...
... Describe what happens to bonds in exothermic and endothermic reactions. Describe how a catalyst works, in terms of energy. Give an advantage and a disadvantage of hydrogen fuel cells. What colour flame will the following ions burn with; K+, Na+, Ca2+, Ba2+, Li+? What colour precipitate is forme ...
KFUPM
... In the synthesis of aspartame, the starting materials are a racemic mixture (equal quantities of both isomers) of phenylalanine, and aspartic acid. Only the L isomer of phenylalanine is deisred for use. This L isomer may be separated from the D isomer by a chemical pretreatment, followed by a reacti ...
... In the synthesis of aspartame, the starting materials are a racemic mixture (equal quantities of both isomers) of phenylalanine, and aspartic acid. Only the L isomer of phenylalanine is deisred for use. This L isomer may be separated from the D isomer by a chemical pretreatment, followed by a reacti ...
enzymatic resolution of a racemic mixture by acylation in
... column coated with a 0.25 Rm thickness film of 20 % 2,3-dimethyl-6-tert-butyldimethylsilyl)ß-cyclodextrin dissolved in BGB-15 from BGB Analytik AG. RESULTS AND DISCUSSION Both enzymes readily catalyzed the hydrolysis of succinic anhydride that was then followed by the reaction between succinic acid ...
... column coated with a 0.25 Rm thickness film of 20 % 2,3-dimethyl-6-tert-butyldimethylsilyl)ß-cyclodextrin dissolved in BGB-15 from BGB Analytik AG. RESULTS AND DISCUSSION Both enzymes readily catalyzed the hydrolysis of succinic anhydride that was then followed by the reaction between succinic acid ...
Gizmos: Types of Reactions
... is a substance consisting of one kind of atom, such as aluminum (Al) or oxygen gas (O2). A compound is a substance made of more than one kind of atom, such as water (H2O) or table salt (NaCl). Question: How are chemical reactions classified? 1. Match: Most chemical reactions can be classified as one ...
... is a substance consisting of one kind of atom, such as aluminum (Al) or oxygen gas (O2). A compound is a substance made of more than one kind of atom, such as water (H2O) or table salt (NaCl). Question: How are chemical reactions classified? 1. Match: Most chemical reactions can be classified as one ...
Oxidation of Ethanol, Esters, Polymerization, Amino
... monomers. Condensation polymerisation is a process by which two molecules join together, with the loss of a small molecule which is often water or hydrogen chloride. Types of condensation polymers include polyamides, polyacetals and polyesters. Polyester is created through ester linkages between mon ...
... monomers. Condensation polymerisation is a process by which two molecules join together, with the loss of a small molecule which is often water or hydrogen chloride. Types of condensation polymers include polyamides, polyacetals and polyesters. Polyester is created through ester linkages between mon ...
Protecting Groups Introduction to Carbonyl
... Solving this problem requires a three-step strategy: [1] Convert the OH group into another functional group that does not interfere with the desired reaction. This new blocking group is called a protecting group, and the reaction that creates it is called “protection.” [2] Carry out the desired reac ...
... Solving this problem requires a three-step strategy: [1] Convert the OH group into another functional group that does not interfere with the desired reaction. This new blocking group is called a protecting group, and the reaction that creates it is called “protection.” [2] Carry out the desired reac ...
Ch 26 C-C bond formation
... Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hyd ...
... Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hyd ...
A Diels-Alder Synthesis
... Look-up the "12 Principles" of Green Chemistry. (wikipedia anyone?) Which of these principles are conformed with by our synthetic method? Which of these principles are explicitly violated? ...
... Look-up the "12 Principles" of Green Chemistry. (wikipedia anyone?) Which of these principles are conformed with by our synthetic method? Which of these principles are explicitly violated? ...
Problem Set 2
... Peroxyacylnitrate is one of the components of smog. It is a compound of C, H, N, and O. It has the following percent composition by mass: 19.8% C, 2.59% H, 11.6% N, and 66.0% O. a. Determine the simplest formula of the compound. b. What is the molecular formula of the compound if its molar mass is a ...
... Peroxyacylnitrate is one of the components of smog. It is a compound of C, H, N, and O. It has the following percent composition by mass: 19.8% C, 2.59% H, 11.6% N, and 66.0% O. a. Determine the simplest formula of the compound. b. What is the molecular formula of the compound if its molar mass is a ...
Review Questions
... number, spontaneity rule, protective coating to prevent rust, Cathodic protection, anode, cathode, standard cell potential Describe the structure and function of the common dry cell. Give an example using MnO2, NH4Cl and Zn electrode Predicting Spontaneity in galvanic and electrolytic cells Ca ...
... number, spontaneity rule, protective coating to prevent rust, Cathodic protection, anode, cathode, standard cell potential Describe the structure and function of the common dry cell. Give an example using MnO2, NH4Cl and Zn electrode Predicting Spontaneity in galvanic and electrolytic cells Ca ...
Chapter 21 The Chemistry of Carboxylic Acid Derivatives
... The reaction of a Grignard reagent with ethyl formate gives a secondary alcohol in which the two alkyl groups at the a-carbon are identical. ...
... The reaction of a Grignard reagent with ethyl formate gives a secondary alcohol in which the two alkyl groups at the a-carbon are identical. ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.