Chapter 21 The Chemistry of Carboxylic Acid Derivatives
... The reaction of a Grignard reagent with ethyl formate gives a secondary alcohol in which the two alkyl groups at the a-carbon are identical. ...
... The reaction of a Grignard reagent with ethyl formate gives a secondary alcohol in which the two alkyl groups at the a-carbon are identical. ...
Quick Lab: Preparation of Esters
... a. Made from alcohols and non-organic acids b. Used to make perfumes and flavorings c. Poisonous d. Foul smelling compounds 2. Esterification is a a. Condensation reaction b. Irreversible reaction c. Addition reaction d. Neutralization reaction 3. What is the general formula for an alcohol a. CnH2n+ ...
... a. Made from alcohols and non-organic acids b. Used to make perfumes and flavorings c. Poisonous d. Foul smelling compounds 2. Esterification is a a. Condensation reaction b. Irreversible reaction c. Addition reaction d. Neutralization reaction 3. What is the general formula for an alcohol a. CnH2n+ ...
Lesmahagow High School CfE Advanced Higher Chemistry Unit 2
... The addition of water to an alkene is done in the presence of an acid catalyst. Again this process can produce more than one product. The more likely product of the reaction can be predicted by using Markovnikov’s rule. When water adds across an asymmetrical double bond the major product formed is t ...
... The addition of water to an alkene is done in the presence of an acid catalyst. Again this process can produce more than one product. The more likely product of the reaction can be predicted by using Markovnikov’s rule. When water adds across an asymmetrical double bond the major product formed is t ...
O 2
... With so many reactions occurring in our lives the Chemist needs a way to help organize them into some sort of manageable scheme. There are 5 general types of chemical reactions that Make up this scheme ...
... With so many reactions occurring in our lives the Chemist needs a way to help organize them into some sort of manageable scheme. There are 5 general types of chemical reactions that Make up this scheme ...
- EdShare - University of Southampton
... The questions in this resource are based on past exam questions, and are designed to be challenging. The purpose of this resource is as a self-assessment exercise, which you can then look back over using specially made talking mark schemes, explaining the answers to the problems, the process in answ ...
... The questions in this resource are based on past exam questions, and are designed to be challenging. The purpose of this resource is as a self-assessment exercise, which you can then look back over using specially made talking mark schemes, explaining the answers to the problems, the process in answ ...
- EdShare - University of Southampton
... The questions in this resource are based on past exam questions, and are designed to be challenging. The purpose of this resource is as a self-assessment exercise, which you can then look back over using specially made talking mark schemes, explaining the answers to the problems, the process in answ ...
... The questions in this resource are based on past exam questions, and are designed to be challenging. The purpose of this resource is as a self-assessment exercise, which you can then look back over using specially made talking mark schemes, explaining the answers to the problems, the process in answ ...
IntroRedoxDCIAns
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
Introduction to Oxidation Reduction
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
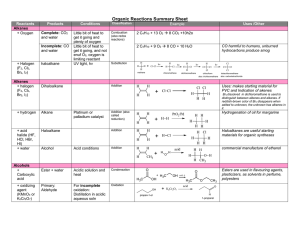
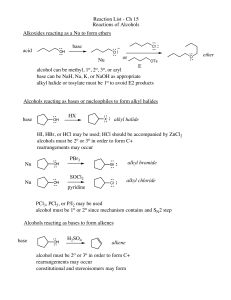
Reaction List - Ch 15 Reactions of Alcohols Alkoxides reacting as a
... alcohol can be methyl, 1o, 2o, 3o, or aryl base can be NaH, Na, K, or NaOH as appropriate alkyl halide or tosylate must be 1o to avoid E2 products Alcohols reacting as bases or nucleophiles to form alkyl halides base ...
... alcohol can be methyl, 1o, 2o, 3o, or aryl base can be NaH, Na, K, or NaOH as appropriate alkyl halide or tosylate must be 1o to avoid E2 products Alcohols reacting as bases or nucleophiles to form alkyl halides base ...
Alkenes from Alcohols
... place four to five drops of your product. Do not confuse the test tubes. Take one tube and add 10 - 15 drops of a solution of bromine in methylene chloride, drop by drop. Repeat with the second tube. Record your observations for each test tube. Analyze the meaning of your results and comment on this ...
... place four to five drops of your product. Do not confuse the test tubes. Take one tube and add 10 - 15 drops of a solution of bromine in methylene chloride, drop by drop. Repeat with the second tube. Record your observations for each test tube. Analyze the meaning of your results and comment on this ...
CfE Higher Chemistry Unit 2: Nature‟s Chemistry Esters, Fats and
... 17. When vegetable oils are hydrolysed, mixtures of fatty acids are obtained. The fatty acids can be classified by their degree of unsaturation. The table below shows the composition of each of the mixtures of fatty acids obtained when palm oil and olive oil were hydrolysed. ...
... 17. When vegetable oils are hydrolysed, mixtures of fatty acids are obtained. The fatty acids can be classified by their degree of unsaturation. The table below shows the composition of each of the mixtures of fatty acids obtained when palm oil and olive oil were hydrolysed. ...
Chemical Reactions
... reactants and products. 2. The relative masses of the reactants and products of a chemical reaction can be determined from the reaction’s coefficients. 3. The reverse reaction for a chemical equation has the same relative amounts of substances as the forward reaction. ...
... reactants and products. 2. The relative masses of the reactants and products of a chemical reaction can be determined from the reaction’s coefficients. 3. The reverse reaction for a chemical equation has the same relative amounts of substances as the forward reaction. ...
Carbohydrates important reactions
... Ruff shortening of glucose gave arabinose, and Kiliani-Fischer synthesis applied to arabinose gave a mixture of glucose and mannose. ...
... Ruff shortening of glucose gave arabinose, and Kiliani-Fischer synthesis applied to arabinose gave a mixture of glucose and mannose. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 19. How will you synthesise the following compounds from benzaldehyde? a) Mandelic acid b) m-Benzaldehydesulphonic acid c) Toluene (2+2+1) 20. Write a note on acidity of substituted benzoic acid. 21. How will you prepare the following compounds from benzenediazonium chloride? a) p-aminoazobenzene b) ...
... 19. How will you synthesise the following compounds from benzaldehyde? a) Mandelic acid b) m-Benzaldehydesulphonic acid c) Toluene (2+2+1) 20. Write a note on acidity of substituted benzoic acid. 21. How will you prepare the following compounds from benzenediazonium chloride? a) p-aminoazobenzene b) ...
New aniline photocage for carboxylic acids
... important topic of current research in organic photochemistry. Wang et al. have recently reported an interesting example of photocage for alcohols, based on m-hydroxyaniline ethers.[5] We decided to further develop this type of protecting group and expand their application for the protection of carb ...
... important topic of current research in organic photochemistry. Wang et al. have recently reported an interesting example of photocage for alcohols, based on m-hydroxyaniline ethers.[5] We decided to further develop this type of protecting group and expand their application for the protection of carb ...
Final Review: L17-25
... element carries a negative oxidation number equal to its charge as an anion. 6. In an ionic compound, the sum of the oxidation numbers for each of the atoms in the compound is equal to 0. 7. In a polyatomic ion, the sum of the oxidation numbers for each of the atoms in the compound is equal to the i ...
... element carries a negative oxidation number equal to its charge as an anion. 6. In an ionic compound, the sum of the oxidation numbers for each of the atoms in the compound is equal to 0. 7. In a polyatomic ion, the sum of the oxidation numbers for each of the atoms in the compound is equal to the i ...
1.5.16(Chem) - mrcarlsonschemistryclass
... Compounds • Atoms bonded together with an IONIC bond are called ionic compounds. • An ionic bond is a METAL bonded with a NONMETAL. • Draw the crystal lattice structure for sodium chloride: ...
... Compounds • Atoms bonded together with an IONIC bond are called ionic compounds. • An ionic bond is a METAL bonded with a NONMETAL. • Draw the crystal lattice structure for sodium chloride: ...
CHEMISTRY 1000
... The alkoxide ion could alternately have been prepared by reacting the alcohol with sodium or potassium. This is usually done when the alcohol is also the solvent for the reaction. These reactions are analogous to the reactions between alkali metals and water that you studied in CHEM 1000. Do you rem ...
... The alkoxide ion could alternately have been prepared by reacting the alcohol with sodium or potassium. This is usually done when the alcohol is also the solvent for the reaction. These reactions are analogous to the reactions between alkali metals and water that you studied in CHEM 1000. Do you rem ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.