Chapter 12 Packet
... How many grams of HNO3 are needed to dissolve 11.45g of Cu? 21) The reaction of powdered aluminum and iron(II)oxide, 2Al(s) + Fe2O3(s) Al2O3(s) + 2Fe(l) produces so much heat the iron that forms is molten. Because of this, railroads use the reaction to provide molten steel to weld steel rails toge ...
... How many grams of HNO3 are needed to dissolve 11.45g of Cu? 21) The reaction of powdered aluminum and iron(II)oxide, 2Al(s) + Fe2O3(s) Al2O3(s) + 2Fe(l) produces so much heat the iron that forms is molten. Because of this, railroads use the reaction to provide molten steel to weld steel rails toge ...
14875-46074-1
... As seen by the results from Table 1, the isolated yields of 1a-6a are in between 90% and 97 % and the reactions completed after 5 to 12 minutes. A comparative observation can be made with Wang et al. [14] who heated to a temperature of 150 °C under pressure for the deprotection of N-Boc amines, wher ...
... As seen by the results from Table 1, the isolated yields of 1a-6a are in between 90% and 97 % and the reactions completed after 5 to 12 minutes. A comparative observation can be made with Wang et al. [14] who heated to a temperature of 150 °C under pressure for the deprotection of N-Boc amines, wher ...
Problems - Department of Chemistry HKU
... where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study ...
... where p0 is the initial pressure and p is the final pressure of cyclopropane. What is the order and rate constant for the reaction under these conditions? 21.10 The addition of hydrogen halides to alkenes has played a fundamental role in the investigation of organic reaction mechanisms. In one study ...
Lesson Plan: Synthesis of Isopentyl Acetate
... The 1R and 2R group side chains can be linear or branch-chain aliphatic or aromatic groups and they can be the same or dissimilar groups. The ester functional group can be synthesized by many methods. The simplest approach is Fisher esterification. By this method, esters are produced by refluxing a ...
... The 1R and 2R group side chains can be linear or branch-chain aliphatic or aromatic groups and they can be the same or dissimilar groups. The ester functional group can be synthesized by many methods. The simplest approach is Fisher esterification. By this method, esters are produced by refluxing a ...
9. Balancing Equations
... • Use coefficients to balance the numbers of atoms on each side; Na2O + BaCl2 - NaCl + BaO How many sodiums on the left? 2; how many on the right/ 1; put a coefficient 2 in front of the one on the right. How many oxygens on the left/ on the right/, etc. Na2O + BaCl2 - 2NaCl + BaO ...
... • Use coefficients to balance the numbers of atoms on each side; Na2O + BaCl2 - NaCl + BaO How many sodiums on the left? 2; how many on the right/ 1; put a coefficient 2 in front of the one on the right. How many oxygens on the left/ on the right/, etc. Na2O + BaCl2 - 2NaCl + BaO ...
Final Review 2006
... c. disassociates into ions. b. forms a precipitate. d. reacts with the water. ____ 44. The formulas for the products of the reaction between sodium hydroxide and sulfuric acid are ____. a. Na2SO4 and H2O. c. SI4 and Na2O. b. NaSO4 and H2O. d. S + O2 and Na. ____ 45. An element in the activity series ...
... c. disassociates into ions. b. forms a precipitate. d. reacts with the water. ____ 44. The formulas for the products of the reaction between sodium hydroxide and sulfuric acid are ____. a. Na2SO4 and H2O. c. SI4 and Na2O. b. NaSO4 and H2O. d. S + O2 and Na. ____ 45. An element in the activity series ...
Lesson 14.1 Acid
... The formation of complex ions can also be looked at as a Lewis acid-base reaction. Complex ions are formed when a metal bonds to electron paris from molecules such as H2O or NH3, or from anions such as CN-. An example of a complex ion is Al(H2O)63+. Hydrated ions like this are present in compounds ...
... The formation of complex ions can also be looked at as a Lewis acid-base reaction. Complex ions are formed when a metal bonds to electron paris from molecules such as H2O or NH3, or from anions such as CN-. An example of a complex ion is Al(H2O)63+. Hydrated ions like this are present in compounds ...
Dear Chemistry Student, I am excited that you have chosen to
... I am excited that you have chosen to challenge yourself by taking on the rigors of AP Chemistry here at Cathedral Catholic High School. In order to prepare you for the expected performance outcomes of the course, every one will be expected to complete a Summer Independent Study Program. Since studen ...
... I am excited that you have chosen to challenge yourself by taking on the rigors of AP Chemistry here at Cathedral Catholic High School. In order to prepare you for the expected performance outcomes of the course, every one will be expected to complete a Summer Independent Study Program. Since studen ...
1.Hydrocarbons contain only hydrogen and carbon. Which of the
... constant of the backward reaction is Kb. What is the relationship between Kf and Kb? (Points : 4) Kf = Kb Kb = 1 + Kf Kf = 1/Kb ...
... constant of the backward reaction is Kb. What is the relationship between Kf and Kb? (Points : 4) Kf = Kb Kb = 1 + Kf Kf = 1/Kb ...
The Hydroxylation of Aromatic Nitro Compounds by Alkalies
... are not solely responBible tor the formation of nitrites. for re-treating the unreacted m-dlnitrobenzene with alkali gave the same effect. Lobry de Bruyn (1894) reported this hydrolysis of nitrobenzene, but at least one major reference book on organic chemistry (Whitmore 1937, p. 730) states that a ...
... are not solely responBible tor the formation of nitrites. for re-treating the unreacted m-dlnitrobenzene with alkali gave the same effect. Lobry de Bruyn (1894) reported this hydrolysis of nitrobenzene, but at least one major reference book on organic chemistry (Whitmore 1937, p. 730) states that a ...
Level 3 Chemistry (91391) 2013
... (ii) Link the structure of enantiomers to a physical property that can be used to distinguish them from non-optically active molecules. ...
... (ii) Link the structure of enantiomers to a physical property that can be used to distinguish them from non-optically active molecules. ...
The characteristic reaction of aromatic rings
... Halo Substitutents are weakly deactivating but are also ortho, para directors l In electrophilic substitution of chlorobenzene, the ortho ...
... Halo Substitutents are weakly deactivating but are also ortho, para directors l In electrophilic substitution of chlorobenzene, the ortho ...
Reaction Rate review questions
... Show that the sum of the two steps in the reaction mechanism is the same as the overall equation for the reaction. What is the rate-determining step? Explain. First step is the slowest so it is the rate determining step. Identify any intermediates or catalysts. F is an intermediate, no catalyst. Pre ...
... Show that the sum of the two steps in the reaction mechanism is the same as the overall equation for the reaction. What is the rate-determining step? Explain. First step is the slowest so it is the rate determining step. Identify any intermediates or catalysts. F is an intermediate, no catalyst. Pre ...
Science24-UnitA-Section3.1-3.2
... a. solid zinc + hydrochloric acid → hydrogen gas + zinc chloride ______________________________________________________________________________________ b. ammonium nitrate + sodium hydroxide → ammonium hydroxide + sodium nitrate _______________________________________________________________________ ...
... a. solid zinc + hydrochloric acid → hydrogen gas + zinc chloride ______________________________________________________________________________________ b. ammonium nitrate + sodium hydroxide → ammonium hydroxide + sodium nitrate _______________________________________________________________________ ...
Organic Compounds containing Oxygen
... Tischenko Reaction : All aldehyde in presence of aluminium ethoxide, Al(OC2H5)3 can be simultaneously oxidised (to acid) and reduced (to alcohols) to form ester. This is called Tischenko reaction and is ...
... Tischenko Reaction : All aldehyde in presence of aluminium ethoxide, Al(OC2H5)3 can be simultaneously oxidised (to acid) and reduced (to alcohols) to form ester. This is called Tischenko reaction and is ...
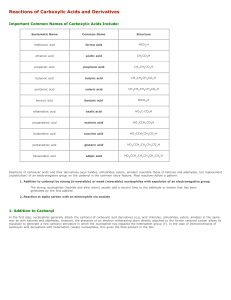
Reactions of Carboxylic Acids and Derivatives
... acetamide [CH 3 CONH2 ] into N,N-dimethylacetamide [CH 3 CON(CH 3 ) 2 ] , it is necessary to hydrolyze first to the carboxylic acid with water and acid (or to the carboxylate salt with water and base (e.g. KOH) ), next convert to the acid chloride with thionyl chloride, and then treat with dimethyl ...
... acetamide [CH 3 CONH2 ] into N,N-dimethylacetamide [CH 3 CON(CH 3 ) 2 ] , it is necessary to hydrolyze first to the carboxylic acid with water and acid (or to the carboxylate salt with water and base (e.g. KOH) ), next convert to the acid chloride with thionyl chloride, and then treat with dimethyl ...
Summary
... with which film characteristics such as thickness and elemental composition can be manipulated. Ideal CVD precursors must be volatile, however, they must also strike a balance between stability and reactivity. Precursors must be volatile to allow the compound to vaporize, yet they must be stable up ...
... with which film characteristics such as thickness and elemental composition can be manipulated. Ideal CVD precursors must be volatile, however, they must also strike a balance between stability and reactivity. Precursors must be volatile to allow the compound to vaporize, yet they must be stable up ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.