Heats of Formation WS
... 7. The Ostwald process for the commercial production of nitric acid from ammonia and oxygen involves the following steps: 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g) 2 NO (g) + O2 (g) 2 NO2 (g) 3 NO2 (g) + H2O (l) 2 HNO3 (aq) + NO (g) [a] Use the values of ∆Hfº to calculate the value of ∆Hº for ...
... 7. The Ostwald process for the commercial production of nitric acid from ammonia and oxygen involves the following steps: 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g) 2 NO (g) + O2 (g) 2 NO2 (g) 3 NO2 (g) + H2O (l) 2 HNO3 (aq) + NO (g) [a] Use the values of ∆Hfº to calculate the value of ∆Hº for ...
Unit 13 Organic Chem AE
... 1) Carbon forms 4 covalent bonds which may be single, double or triple. This is due to the four unpaired valence electrons that carbon has in its ground state. These four bonds have a TETRAHEDRAL arrangement. 2) Most organic compounds are nonpolar or weakly polar. This means that most are held toget ...
... 1) Carbon forms 4 covalent bonds which may be single, double or triple. This is due to the four unpaired valence electrons that carbon has in its ground state. These four bonds have a TETRAHEDRAL arrangement. 2) Most organic compounds are nonpolar or weakly polar. This means that most are held toget ...
element
... elements. Write their symbol on your white board and hold it up when done. (Let’s see who knows the Periodic Table BEST!) ...
... elements. Write their symbol on your white board and hold it up when done. (Let’s see who knows the Periodic Table BEST!) ...
2005 - NESACS
... 75. A student pipetted five 25.00-milliliter samples of hydrochloric acid and transferred each sample to an Erlenmeyer flask, diluted each with distilled water, and added a few drops of phenolphthalein to each. Each sample was then titrated with a sodium hydroxide solution to the appearance of the f ...
... 75. A student pipetted five 25.00-milliliter samples of hydrochloric acid and transferred each sample to an Erlenmeyer flask, diluted each with distilled water, and added a few drops of phenolphthalein to each. Each sample was then titrated with a sodium hydroxide solution to the appearance of the f ...
blank lecture 11
... • 1-propene-3-thiol and 3,3-di-(1-propenyl)disulfide – • trans-2-butene-1-thiol, 3-methyl-1-butanethiol, and methyl1-(trans-2-butenyl)disulfide – ...
... • 1-propene-3-thiol and 3,3-di-(1-propenyl)disulfide – • trans-2-butene-1-thiol, 3-methyl-1-butanethiol, and methyl1-(trans-2-butenyl)disulfide – ...
Alkanes – Molecules w/o functional Groups
... – Ka = K[H20] = [H3O+][A-]/[HA] – pKa = -log Ka ( pKa = pH + pA- -pHA) – pKa = pH where 50% of acid is dissociated [A] = [HA] – “weak acids” pKa > 4 ...
... – Ka = K[H20] = [H3O+][A-]/[HA] – pKa = -log Ka ( pKa = pH + pA- -pHA) – pKa = pH where 50% of acid is dissociated [A] = [HA] – “weak acids” pKa > 4 ...
File
... Nitrogen dioxide + water(l) nitric acid + nitrogen monoxide Nitrogen dioxide(g) + water(l) nitric acid(aq) + nitrogen monoxide(g) NO2(g) + H2O(l) HNO3(aq) + NO(g) 3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g) ...
... Nitrogen dioxide + water(l) nitric acid + nitrogen monoxide Nitrogen dioxide(g) + water(l) nitric acid(aq) + nitrogen monoxide(g) NO2(g) + H2O(l) HNO3(aq) + NO(g) 3 NO2(g) + H2O(l) 2 HNO3(aq) + NO(g) ...
Functional Groups
... 2. Identify the groups (other than H) that are joined to the longest chain. Add the names of these groups as a prefix along with di-, tri-, etc. to indicate the number of each group. CH3-CH-CH2-CH-CH3 ...
... 2. Identify the groups (other than H) that are joined to the longest chain. Add the names of these groups as a prefix along with di-, tri-, etc. to indicate the number of each group. CH3-CH-CH2-CH-CH3 ...
EXPERIMENT 4 (Organic Chemistry II) Pahlavan/Cherif
... Zinc chloride (ZnCl2) is a catalyst for this reaction, which is called the Lucas test. The resultant alkyl chloride is insoluble in water and separates from the Lucas reagent (ZnCl2 in concentrated HCl), forming a cloudy mixture. Alcohols react at different rates, depending upon their structure. Ter ...
... Zinc chloride (ZnCl2) is a catalyst for this reaction, which is called the Lucas test. The resultant alkyl chloride is insoluble in water and separates from the Lucas reagent (ZnCl2 in concentrated HCl), forming a cloudy mixture. Alcohols react at different rates, depending upon their structure. Ter ...
Chapter 5-alcohol
... greater number of alkyl groups on the double bond generally predominates. ◦ Required an acid catalyst and heat ...
... greater number of alkyl groups on the double bond generally predominates. ◦ Required an acid catalyst and heat ...
PDF 4/page - (canvas.brown.edu).
... α-D-Glucopyranose has 4 equatorial and 1 axial substitutions on the pyranose ring whereas β-DGlucopyranose has 5 equatorial substituents on the pyranose ring. Minimization of steric hindrance favors equatorial positions for the highest number of pyranose substituents. The anomeric effect involving s ...
... α-D-Glucopyranose has 4 equatorial and 1 axial substitutions on the pyranose ring whereas β-DGlucopyranose has 5 equatorial substituents on the pyranose ring. Minimization of steric hindrance favors equatorial positions for the highest number of pyranose substituents. The anomeric effect involving s ...
Structure of Organic Compounds Infra
... The number of double bond equivalents in a molecule tells us how many double bonds and/or rings are present in the molecule. A value of zero tells us the molecule in question has no double bonds, no triple bonds, and/or no rings. A value of one tells us that the molecule in question has one d ...
... The number of double bond equivalents in a molecule tells us how many double bonds and/or rings are present in the molecule. A value of zero tells us the molecule in question has no double bonds, no triple bonds, and/or no rings. A value of one tells us that the molecule in question has one d ...
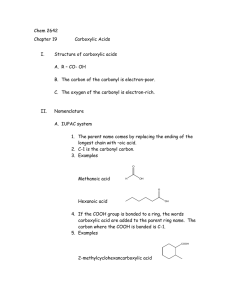
Carboxylic Acids and Their Derivatives
... • Ester hydrolysis can be speeded up by using acid as a catalyst. • H+ works by first protonating the carbonyl oxygen to make it more reactive • Like the reverse rxn (esterification) hydrolysis is an equilibrium process with a tetrahedral intermediate • Both OH- and OR- groups are equally likely to ...
... • Ester hydrolysis can be speeded up by using acid as a catalyst. • H+ works by first protonating the carbonyl oxygen to make it more reactive • Like the reverse rxn (esterification) hydrolysis is an equilibrium process with a tetrahedral intermediate • Both OH- and OR- groups are equally likely to ...
Bal Equations notes.cwk (WP)
... When hydrocarbons such as gasoline, methane, propane and sucrose are burned (combusted) they always produce energy plus carbon dioxide gas and water vapor. The act of burning is actually just a rapid reaction with oxygen. The equations are just the compound to be burned plus oxygen to produce carbon ...
... When hydrocarbons such as gasoline, methane, propane and sucrose are burned (combusted) they always produce energy plus carbon dioxide gas and water vapor. The act of burning is actually just a rapid reaction with oxygen. The equations are just the compound to be burned plus oxygen to produce carbon ...
Exam I - Chemistry With BT
... instead of writing everything in their lab note-books, they tabulate it on a sheet of paper, which subsequently goes missing. They do remember the H(combustion) values to be different for their samples. In order to help the researchers, please construct the energy diagram below filling the boxes wi ...
... instead of writing everything in their lab note-books, they tabulate it on a sheet of paper, which subsequently goes missing. They do remember the H(combustion) values to be different for their samples. In order to help the researchers, please construct the energy diagram below filling the boxes wi ...
Section II - School District 27J
... Tungsten metal may be prepared by reducing WO3 with H2 gas. How many grams of tungsten may be prepared from 0.0500 mol of WO3 with excess H2? (Water is also a product of this reaction.) (A) 5.58 g (B) 0.500 g (C) 9.19 g (D) 184 g (E) 18.4 g ...
... Tungsten metal may be prepared by reducing WO3 with H2 gas. How many grams of tungsten may be prepared from 0.0500 mol of WO3 with excess H2? (Water is also a product of this reaction.) (A) 5.58 g (B) 0.500 g (C) 9.19 g (D) 184 g (E) 18.4 g ...
Chemical Reactions (L1)
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
... (BrINClHOF) For example, Oxygen is O2 as an element. In a compound, it can’t be a diatomic element because it’s not an element anymore, it’s a compound! ...
Full answers
... 4 isomers: there are 2 pairs of enantiomers: Each isomer has 1 enantiomer and 2 diastereoisomers Give the products formed when methylphenidate is hydrolysed with 4 M HCl. ...
... 4 isomers: there are 2 pairs of enantiomers: Each isomer has 1 enantiomer and 2 diastereoisomers Give the products formed when methylphenidate is hydrolysed with 4 M HCl. ...
Answers - Final Exam 2013
... related to reagents that have been covered in CHEM 203. On the basis of structural analogy, indicate the probable use of each of them (write your answers in the boxes): ...
... related to reagents that have been covered in CHEM 203. On the basis of structural analogy, indicate the probable use of each of them (write your answers in the boxes): ...
unit 4 practice
... 19. According to this information, as the temperature of the system increases, the equilibrium shifts A. left, and the reaction is exothermic B. left and the reaction is endothermic C. right and th ...
... 19. According to this information, as the temperature of the system increases, the equilibrium shifts A. left, and the reaction is exothermic B. left and the reaction is endothermic C. right and th ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.