CHEMISTRY 314-01 MIDTERM # 3 – answer key December 03
... 2) H2O O 21. (5 pts) The two isomeric carboxylic acids (C5H10O2), whose 1H NMR spectra are shown below, were produced using the malonic ester synthesis. a. Propose structures for the acids. b. What alkyl halide(s) was (were) used in each case? ...
... 2) H2O O 21. (5 pts) The two isomeric carboxylic acids (C5H10O2), whose 1H NMR spectra are shown below, were produced using the malonic ester synthesis. a. Propose structures for the acids. b. What alkyl halide(s) was (were) used in each case? ...
unit c3 – chemistry in action checklist
... Demonstrate an understanding that some areas of the country have dissolved calcium or magnesium ions in their tap water and that the presence of these ions makes the water hard Describe problems caused by hard water, including: a it does not easily form a lather with soap b it reacts with soap to fo ...
... Demonstrate an understanding that some areas of the country have dissolved calcium or magnesium ions in their tap water and that the presence of these ions makes the water hard Describe problems caused by hard water, including: a it does not easily form a lather with soap b it reacts with soap to fo ...
Test - Regents
... (3) 33.6 L of CH4(g) (1) 11.2 L of H2(g) (2) 22.4 L of CO2(g) (4) 44.8 L of O2(g) ...
... (3) 33.6 L of CH4(g) (1) 11.2 L of H2(g) (2) 22.4 L of CO2(g) (4) 44.8 L of O2(g) ...
Brominations and Alkene Synthesis CHM 233 Review
... • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearrangements - we will use this information later! ...
... • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearrangements - we will use this information later! ...
CHM 331 : General Organic Chemistry
... • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearrangements - we will use this information later! ...
... • note new type of structure for indicating stereochemistry on rings, trans- in this case • NOTE, because of bromonium ion intermediate (no "free" carbocation) there are no rearrangements - we will use this information later! ...
Example: Writing a Thermochemical Equation
... Consider the reaction of hydrogen and oxygen to produce water. If the product is water vapor, 2 mol of H2 burn to release 483.7 kJ of heat. 2H2(g) + O2(g) → 2H2O(g); ∆H = -483.7 kJ On the other hand, if the product is liquid water, the heat released is 571.7 kJ. 2H2(g) + O2(g) → 2H2O(l); ∆H = -571. ...
... Consider the reaction of hydrogen and oxygen to produce water. If the product is water vapor, 2 mol of H2 burn to release 483.7 kJ of heat. 2H2(g) + O2(g) → 2H2O(g); ∆H = -483.7 kJ On the other hand, if the product is liquid water, the heat released is 571.7 kJ. 2H2(g) + O2(g) → 2H2O(l); ∆H = -571. ...
Precipitation Reactions
... one acidic hydrogen to form a neutral compound (acid). •These acids are called polyprotic (diprotic, triprotic, et cet.) •It is possible to remove only one of the multiple acidic hydrogens. In that case, the created anion is itself acidic. ...
... one acidic hydrogen to form a neutral compound (acid). •These acids are called polyprotic (diprotic, triprotic, et cet.) •It is possible to remove only one of the multiple acidic hydrogens. In that case, the created anion is itself acidic. ...
Learning Guide – Poisons (I)
... Meat turns brown when you cook it. Plants make sugar and oxygen from carbon dioxide and water. “Hot hands” get warm when bent. Old wine turns into vinegar. Paint remover loosens paint so it can be removed. Balancing chemical reactions When we write a chemical reaction, it is important to know how ma ...
... Meat turns brown when you cook it. Plants make sugar and oxygen from carbon dioxide and water. “Hot hands” get warm when bent. Old wine turns into vinegar. Paint remover loosens paint so it can be removed. Balancing chemical reactions When we write a chemical reaction, it is important to know how ma ...
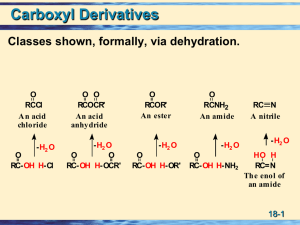
Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution
... Amides having at least one NOH unit can form intermolecular hydrogen bonds with other amide molecules. Compounds of this type have higher melting and boiling points than comparable compounds in which NOH bonds are absent. Hydrogen bonding in amides influences the conformations of proteins. Amides ar ...
... Amides having at least one NOH unit can form intermolecular hydrogen bonds with other amide molecules. Compounds of this type have higher melting and boiling points than comparable compounds in which NOH bonds are absent. Hydrogen bonding in amides influences the conformations of proteins. Amides ar ...
Basic IUPAC Nomenclature V
... direction you first run into the carbon attached to the alcohol is the direction use to number the chain. Other substituents no longer matter only the alcohol determines direction. a. If the numbers are the same go to the next highest priority functional group or substituent and follow its priority ...
... direction you first run into the carbon attached to the alcohol is the direction use to number the chain. Other substituents no longer matter only the alcohol determines direction. a. If the numbers are the same go to the next highest priority functional group or substituent and follow its priority ...
2 C2H6 (g)
... Assume 0.280 mole of N2 and 0.903 mole of H2 are present initially. a) After complete reaction, how many moles of ammonia are produced? b) How many moles of H2 remain? c) How many moles of N2 remain? d) What is the limiting reactant? Question 20 of 28 When calcium carbonate is added to hydrochloric ...
... Assume 0.280 mole of N2 and 0.903 mole of H2 are present initially. a) After complete reaction, how many moles of ammonia are produced? b) How many moles of H2 remain? c) How many moles of N2 remain? d) What is the limiting reactant? Question 20 of 28 When calcium carbonate is added to hydrochloric ...
2.277 October 2005 Mid-Term Test
... All of the statements about the classification of these amino acids are correct EXCEPT: A) Aspartic acid and asparagine are acidic amino acids. B) Alanine and valine are neutral, nonpolar amino acids. C) Serine and glutamine are polar, uncharged amino acids. D) Lysine and arginine are basic amino ac ...
... All of the statements about the classification of these amino acids are correct EXCEPT: A) Aspartic acid and asparagine are acidic amino acids. B) Alanine and valine are neutral, nonpolar amino acids. C) Serine and glutamine are polar, uncharged amino acids. D) Lysine and arginine are basic amino ac ...
T10 AD bioenergetics
... = downhill) for standard conditions: Room temperature, partial pressure of all gases = 100 kPa, all concentrations 1 mol/L. Why is the established value not the one calculated by Bryant for the ...
... = downhill) for standard conditions: Room temperature, partial pressure of all gases = 100 kPa, all concentrations 1 mol/L. Why is the established value not the one calculated by Bryant for the ...
What are the general types of reactions?
... – Mass is not created or destroyed in a chemical reaction – For practical purposes • Same types of atoms before and after a reaction • Same number of each type of atom before and after ...
... – Mass is not created or destroyed in a chemical reaction – For practical purposes • Same types of atoms before and after a reaction • Same number of each type of atom before and after ...
Biodiesel preparation in batch emulgation reactor
... New types of alternative fuels to fossil fuels are being developed nowadays. These new fuels should be environmentally friendly, nontoxic, produced from renewable sources and their price must be competitive with petrol diesel. FAME (fatty acid methyl esters) is one of the alternatives; it is a mixtu ...
... New types of alternative fuels to fossil fuels are being developed nowadays. These new fuels should be environmentally friendly, nontoxic, produced from renewable sources and their price must be competitive with petrol diesel. FAME (fatty acid methyl esters) is one of the alternatives; it is a mixtu ...
File
... melting and boiling points as we go up a series. The reason for this is the increasing London forces as the molecules get larger. • Members of the same homologous series have similar chemical properties and methods of preparation. • The chemical formula increases by CH2 from one member to the next u ...
... melting and boiling points as we go up a series. The reason for this is the increasing London forces as the molecules get larger. • Members of the same homologous series have similar chemical properties and methods of preparation. • The chemical formula increases by CH2 from one member to the next u ...
Ch 12 Alcohols and Thiols
... • Aldehydes and ketones with 4 or less carbons are very soluble in water • Carbonyl can H-bond with water ...
... • Aldehydes and ketones with 4 or less carbons are very soluble in water • Carbonyl can H-bond with water ...
The United States is the largest single consumer of fossil... the U.S. consumes 125 billion gallons of gasoline and 60... Chem 103 Biodiesel Protocol (as taken from Chem 238 Biodiesel Protocol)
... needs is increasing. One such alternative feedstock is vegetable oil. Vegetable oil offers the benefits of a more environmentally sensitive synthetic route for obtaining diesel fuel. This fuel source is commonly known as biodiesel, and can be synthesized on an individual vehicle level or on an indus ...
... needs is increasing. One such alternative feedstock is vegetable oil. Vegetable oil offers the benefits of a more environmentally sensitive synthetic route for obtaining diesel fuel. This fuel source is commonly known as biodiesel, and can be synthesized on an individual vehicle level or on an indus ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.