Journal of Molecular Catalysis A: Chemical Enhancing
... reaction. Encouraged by these results, we next set out to investigate the use of sodium iodide and sodium chloride for iodination and chlorination respectively. During chlorination very poor results were obtained and results of iodination were found to be compatible with the previous one (Table 1). ...
... reaction. Encouraged by these results, we next set out to investigate the use of sodium iodide and sodium chloride for iodination and chlorination respectively. During chlorination very poor results were obtained and results of iodination were found to be compatible with the previous one (Table 1). ...
MOLECULAR REPRESENTATIONS AND INFRARED
... b) (C2H5)2CHC≡C(CH2)3CH3 or CH3CH2CH(C2H5)C≡C(CH2)3CH3 d) (CH3)2CHCH2S(CH2)2CH(C2H5)C(CH3)3 ...
... b) (C2H5)2CHC≡C(CH2)3CH3 or CH3CH2CH(C2H5)C≡C(CH2)3CH3 d) (CH3)2CHCH2S(CH2)2CH(C2H5)C(CH3)3 ...
Redox - SAVE MY EXAMS!
... Separate samples of hydrogen peroxide are added to aqueous potassium iodide and to acidified potassium dichromate(VI). The iodide ions are oxidised and dichromate(VI) ions are reduced. ...
... Separate samples of hydrogen peroxide are added to aqueous potassium iodide and to acidified potassium dichromate(VI). The iodide ions are oxidised and dichromate(VI) ions are reduced. ...
2. 2-Isopropyl-5-methylcyclohexanol on carbon skeletal
... 92. The unimolecular substitution reaction (SN1 ) takes place at a maximum speed of alcohols: 1. neo-hexyl; 2. propyl; 3. tert-butyl; 4. benzyl; 93. Stereospecificity are the reactions occurring at the chiral centers of electrophilic substrates alcohols according to the mechanism: 1. SN1; 2. SN2; 3. ...
... 92. The unimolecular substitution reaction (SN1 ) takes place at a maximum speed of alcohols: 1. neo-hexyl; 2. propyl; 3. tert-butyl; 4. benzyl; 93. Stereospecificity are the reactions occurring at the chiral centers of electrophilic substrates alcohols according to the mechanism: 1. SN1; 2. SN2; 3. ...
Aldehydes and ketones
... Compounds containing the carbonyl group • Amides are the first nitrogen-containing organic compounds we’ve seen. In these compounds, the carbonyl group is bound to a nitrogen (an amino group), in addition to either a H-atom or a carbon group (alkyl, cycloalkyl, aromatic). The R’ and R” groups of th ...
... Compounds containing the carbonyl group • Amides are the first nitrogen-containing organic compounds we’ve seen. In these compounds, the carbonyl group is bound to a nitrogen (an amino group), in addition to either a H-atom or a carbon group (alkyl, cycloalkyl, aromatic). The R’ and R” groups of th ...
Jonpostwriteup
... now to the bromosafrole and solvent pour into sep funnel add equal volume water and start adding bicarb and swirl do this carefully watch for fizzing add bicarb until the fizzing stops. separate the bottom layer and discard the top layer aqueous layer. wash once again with water, then brine then tak ...
... now to the bromosafrole and solvent pour into sep funnel add equal volume water and start adding bicarb and swirl do this carefully watch for fizzing add bicarb until the fizzing stops. separate the bottom layer and discard the top layer aqueous layer. wash once again with water, then brine then tak ...
$doc.title
... • Acyl group bonded to X, an electronega3ve atom or leaving group • Includes: X = halide (acid halides), acyloxy (anhydrides), alkoxy (esters), amine (amides), thiolate (thioesters), phosphate (acyl ...
... • Acyl group bonded to X, an electronega3ve atom or leaving group • Includes: X = halide (acid halides), acyloxy (anhydrides), alkoxy (esters), amine (amides), thiolate (thioesters), phosphate (acyl ...
workbook Chem (WP)
... 1. consist of a metal and a nonmetal (cation and an anion) 2. charges must balance in the final formula to form a neutral compound 3. List the characteristics of an ionic compound. 4. Write the formula for the following: a. lithium chloride b. magnesium fluoride c. scandium sulfide d. calcium nitrid ...
... 1. consist of a metal and a nonmetal (cation and an anion) 2. charges must balance in the final formula to form a neutral compound 3. List the characteristics of an ionic compound. 4. Write the formula for the following: a. lithium chloride b. magnesium fluoride c. scandium sulfide d. calcium nitrid ...
Comparing Free Energies
... Eq. (5.5), imply that chemical reactions will occur in the direction for which DGrxn is negative (DGrxn < 0), this is, in the direction in which the Gibbs free energy of the system decreases. The more negative the change in Gibbs free energy, the more favored the chemical reaction and the larger the ...
... Eq. (5.5), imply that chemical reactions will occur in the direction for which DGrxn is negative (DGrxn < 0), this is, in the direction in which the Gibbs free energy of the system decreases. The more negative the change in Gibbs free energy, the more favored the chemical reaction and the larger the ...
Document
... A functional group is the site of most chemical reactivity of a molecule Alkanes do not have a functional groups a) Alkyl group (R-): obtained by removing a hydrogen atom from an alkane (CnH2n+2): ...
... A functional group is the site of most chemical reactivity of a molecule Alkanes do not have a functional groups a) Alkyl group (R-): obtained by removing a hydrogen atom from an alkane (CnH2n+2): ...
Example of Lab Notebook
... to the theoretical value of 113–115oC. Although reasonably pure, the wider range and depressed values indicate the presence of impurities. Improper drying and removal of water could account for this result. In addition, incomplete washing the product during the filtration process could have allowed ...
... to the theoretical value of 113–115oC. Although reasonably pure, the wider range and depressed values indicate the presence of impurities. Improper drying and removal of water could account for this result. In addition, incomplete washing the product during the filtration process could have allowed ...
Document
... If 4.0 mol N2 and 1.0 mol O2 are mixed in a 5.0 L container, what are all equilibrium concentrations? ...
... If 4.0 mol N2 and 1.0 mol O2 are mixed in a 5.0 L container, what are all equilibrium concentrations? ...
File
... Using the data from Experiment 1, determine the concentration of the substances used and the rate constant for the reaction including its units. ...
... Using the data from Experiment 1, determine the concentration of the substances used and the rate constant for the reaction including its units. ...
functional groups
... phosphorus bound to four oxygen atoms (three with single bonds and one with a double bond). • A phosphate group connects to the carbon backbone via one of its oxygen atoms. • Phosphate groups are anions with two negative charges as two protons have dissociated from the oxygen atoms. • One function o ...
... phosphorus bound to four oxygen atoms (three with single bonds and one with a double bond). • A phosphate group connects to the carbon backbone via one of its oxygen atoms. • Phosphate groups are anions with two negative charges as two protons have dissociated from the oxygen atoms. • One function o ...
AP CHEMISTRY SUMMER ASSIGNMENT
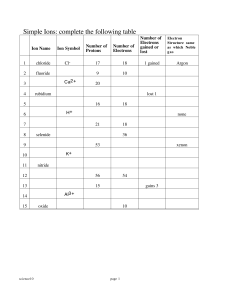
... Cations: ions that have lost electrons and gained a positive charge Anions: ions that have gained electrons and gained a negative charge To calculate the number of protons, neutrons or electrons in any atom or ion: Protons = atomic number Neutrons = mass number – atomic number Electrons = number of ...
... Cations: ions that have lost electrons and gained a positive charge Anions: ions that have gained electrons and gained a negative charge To calculate the number of protons, neutrons or electrons in any atom or ion: Protons = atomic number Neutrons = mass number – atomic number Electrons = number of ...
Chapter 22/23-Organic Chemistry
... 2. Draw the following organic compounds: a. 2,4,4-trimethyl-1-pentene b. 1,2,3-triphenylheptane c. 1,3-dibromo-2-chloro-5-methylcylcohexane d. 3,3-difluoro-1,4-pentadiene e. 4–ethyl–2,6–dimethyl–2–heptene. f. 1,1-dichlorobenzene 3. Based on what you know about the ability of a carbon-carbon double b ...
... 2. Draw the following organic compounds: a. 2,4,4-trimethyl-1-pentene b. 1,2,3-triphenylheptane c. 1,3-dibromo-2-chloro-5-methylcylcohexane d. 3,3-difluoro-1,4-pentadiene e. 4–ethyl–2,6–dimethyl–2–heptene. f. 1,1-dichlorobenzene 3. Based on what you know about the ability of a carbon-carbon double b ...
File
... The reaction of methane and water is one way to prepare hydrogen. CH4(g) + 2 H2O(g) CO2(g) + 4 H2(g) If 0.320 mol of methane reacts with 0.530 mol of water, what is the limiting reagent? a. CH4(g) c. H2(g) b. CO2(g) d. H2O(g) ...
... The reaction of methane and water is one way to prepare hydrogen. CH4(g) + 2 H2O(g) CO2(g) + 4 H2(g) If 0.320 mol of methane reacts with 0.530 mol of water, what is the limiting reagent? a. CH4(g) c. H2(g) b. CO2(g) d. H2O(g) ...
safe disposal of waste containing nitric acid
... Recent incidents at Yale and institutions around the country that have resulted in injuries serve as a reminder of the hazards of working with nitric acid. Nitric acid is an extremely corrosive acid. It is also a strong oxidizer, which reacts violently with many materials including organic compounds ...
... Recent incidents at Yale and institutions around the country that have resulted in injuries serve as a reminder of the hazards of working with nitric acid. Nitric acid is an extremely corrosive acid. It is also a strong oxidizer, which reacts violently with many materials including organic compounds ...
File - Dr KHALID SHADID
... A characteristic reaction of aldehydes and ketones is one of nucleophilic addition to the carbon-oxygen double bond. Carboxylic acids and their derivatives are characterized by a nueleophilic addition-elimination mechanism that takes place at their acyl (carbonyl) carbon atoms. ...
... A characteristic reaction of aldehydes and ketones is one of nucleophilic addition to the carbon-oxygen double bond. Carboxylic acids and their derivatives are characterized by a nueleophilic addition-elimination mechanism that takes place at their acyl (carbonyl) carbon atoms. ...
Final Exam Review Sheet Chemistry 110a/1998
... how the reaction works. 3. Be able to explain how sodium borohydride can be used to reduce aldehydes and ketones, using an arrow-pushing mechanism to explain how the reaction works. 4. Be able to explain the reactivity differences, i.e., chemoselectivity differences, between LAH and sodium borohydri ...
... how the reaction works. 3. Be able to explain how sodium borohydride can be used to reduce aldehydes and ketones, using an arrow-pushing mechanism to explain how the reaction works. 4. Be able to explain the reactivity differences, i.e., chemoselectivity differences, between LAH and sodium borohydri ...
PPT - mvhs-fuhsd.org
... Enthalpy is an extensive property. hence it is proportional to the amount of reactants and products. e.g. for decomposition of two moles of water twice as much energy is needed as for one mole of water. H for a reaction in the forward direction is equal in size, but opposite in sign, to H for the ...
... Enthalpy is an extensive property. hence it is proportional to the amount of reactants and products. e.g. for decomposition of two moles of water twice as much energy is needed as for one mole of water. H for a reaction in the forward direction is equal in size, but opposite in sign, to H for the ...
Alcohols and Carbonyls
... 1. Fehlings solution contains Cu2+ ions (blue) which form Cu+ ion (orange-red) in the presence of aldehydes. 2. Tollen’s reagent contains Ag+ ions, which form Ag in the presence of aldehydes (silver mirror test) 3. Acidified Potassium Dichromate orange Cr2O72-(aq) to green Cr3(aq) ...
... 1. Fehlings solution contains Cu2+ ions (blue) which form Cu+ ion (orange-red) in the presence of aldehydes. 2. Tollen’s reagent contains Ag+ ions, which form Ag in the presence of aldehydes (silver mirror test) 3. Acidified Potassium Dichromate orange Cr2O72-(aq) to green Cr3(aq) ...
Chapter 5 CHEM 121
... BALANCED CHEMICAL EQUATIONS • A balanced chemical equation is one in which the number of atoms of each element in the reactants is equal to the number of atoms of that same element in the products. • A reaction can be balanced by applying the law of conservation of matter. • Coefficients (in red be ...
... BALANCED CHEMICAL EQUATIONS • A balanced chemical equation is one in which the number of atoms of each element in the reactants is equal to the number of atoms of that same element in the products. • A reaction can be balanced by applying the law of conservation of matter. • Coefficients (in red be ...
Strychnine total synthesis

Strychnine total synthesis in chemistry describes the total synthesis of the complex biomolecule strychnine. The first reported method by the group of Robert Burns Woodward in 1954 is considered a classic in this research field. At the time it formed the natural conclusion to an elaborate process of molecular structure elucidation that started with the isolation of strychnine from the beans of Strychnos ignatii by Pierre Joseph Pelletier and Joseph Bienaimé Caventou in 1818. Major contributors to the entire effort were Sir Robert Robinson with over 250 publications and Hermann Leuchs with another 125 papers in a time span of 40 years. Robinson was awarded the Nobel Prize in Chemistry in 1947 for his work on alkaloids, strychnine included. The process of chemical identification was completed with publications in 1946 by Robinson and later confirmed by Woodward in 1947. X-ray structures establishing the absolute configuration became available between 1947 and 1951 with publications from J. M. Bijvoet and J.H. Robertson .Woodward published a very brief account on the strychnine synthesis in 1954 (just 3 pages) and a lengthy one (42 pages) in 1963.Many more methods exist and reported by the research groups of Magnus, Overman, Kuehne, Rawal, Bosch, Vollhardt, Mori, Shibasaki, Li, Fukuyama Vanderwal and MacMillan. Synthetic (+)-strychnine is also known. Racemic synthesises were published by Padwa in 2007 and in 2010 by Andrade and by Reissig.In his 1963 publication Woodward quoted Sir Robert Robinson who said for its molecular size it is the most complex substance known.