Assignment Sheet
... Explain how structure and bonding of carbon lead to the diversity and number of organic compounds. Compare structural and geometric isomers of organic compounds. Distinguish among the structures of alkanes, alkenes, alkynes, and aromatic hydrocarbons. Write structural formulas and names for alkanes, ...
... Explain how structure and bonding of carbon lead to the diversity and number of organic compounds. Compare structural and geometric isomers of organic compounds. Distinguish among the structures of alkanes, alkenes, alkynes, and aromatic hydrocarbons. Write structural formulas and names for alkanes, ...
Paper - Edexcel
... (d) State two advantages of using reaction 3 to manufacture ethanol rather than reaction 1. ...
... (d) State two advantages of using reaction 3 to manufacture ethanol rather than reaction 1. ...
Identification of Unknown Organic Compounds
... Solubility and Functional Group Tests Each functional group has a particular set of chemical properties that allow it to be identified. Some of these properties can be demonstrated by observing solubility behavior, while others can be seen in chemical reactions that are accompanied by color changes, ...
... Solubility and Functional Group Tests Each functional group has a particular set of chemical properties that allow it to be identified. Some of these properties can be demonstrated by observing solubility behavior, while others can be seen in chemical reactions that are accompanied by color changes, ...
PPT
... • This makes low molecular weight alcohols highly soluble in water. • Hydrogen bonding in a water-methanol solution: ...
... • This makes low molecular weight alcohols highly soluble in water. • Hydrogen bonding in a water-methanol solution: ...
PDF
... • The –OH group is polar and capable of hydrogen bonding. • This makes low molecular weight alcohols highly soluble in water. • Hydrogen bonding in a water-methanol solution: ...
... • The –OH group is polar and capable of hydrogen bonding. • This makes low molecular weight alcohols highly soluble in water. • Hydrogen bonding in a water-methanol solution: ...
Alcohols and Carbonyls
... 1. Fehlings solution contains Cu2+ ions (blue) which form Cu+ ion (orange-red) in the presence of aldehydes. 2. Tollen’s reagent contains Ag+ ions, which form Ag in the presence of aldehydes (silver mirror test) 3. Acidified Potassium Dichromate orange Cr2O72-(aq) to green Cr3(aq) ...
... 1. Fehlings solution contains Cu2+ ions (blue) which form Cu+ ion (orange-red) in the presence of aldehydes. 2. Tollen’s reagent contains Ag+ ions, which form Ag in the presence of aldehydes (silver mirror test) 3. Acidified Potassium Dichromate orange Cr2O72-(aq) to green Cr3(aq) ...
The first practical method for asymmetric epoxidation
... ice/CC14) and 50 mL of 10% aqueous tartaric acid solution was added while stirring; the aqueous layer solidified. After 30 min, the cooling bath was removed and stirring was continued at room temperature for 1 h or until the aqueous layer became clear. After separation of the aqueous layer, the orga ...
... ice/CC14) and 50 mL of 10% aqueous tartaric acid solution was added while stirring; the aqueous layer solidified. After 30 min, the cooling bath was removed and stirring was continued at room temperature for 1 h or until the aqueous layer became clear. After separation of the aqueous layer, the orga ...
Chapter 12 (Complexometric Titration)
... coordinate covalent bond ligand donates both electrons of the electron pair bond ...
... coordinate covalent bond ligand donates both electrons of the electron pair bond ...
CH 14-15 Chapter 14-15 review wkey
... 15. According to collision theory, which of the following factors does NOT influence the rate of reaction? a) collision frequency b) collision energy c) collision orientation d) collision rebound direction e) none of these 16. What distance corresponds to the activation energy for the reaction of X ...
... 15. According to collision theory, which of the following factors does NOT influence the rate of reaction? a) collision frequency b) collision energy c) collision orientation d) collision rebound direction e) none of these 16. What distance corresponds to the activation energy for the reaction of X ...
as a PDF
... highly efficient reagents for the selective stoichiometric oxidation of terminal olefins to methyl ketones a t ambient temperature, and catalysts for the ketonization of terminal olefins by tert-butyl hydroperoxide. The removal of one oxygen atom from the tert-butyl peroxidic group in [ C F ~ C O ~ ...
... highly efficient reagents for the selective stoichiometric oxidation of terminal olefins to methyl ketones a t ambient temperature, and catalysts for the ketonization of terminal olefins by tert-butyl hydroperoxide. The removal of one oxygen atom from the tert-butyl peroxidic group in [ C F ~ C O ~ ...
Chemical vapour deposition, CVD
... Mainly thin film application One of the most important methods for preparing thin films and coatings •Protection (corrosion, wear…) •Optical properties •Electronic properties •Magnetic properties •Decoration •New properties ...
... Mainly thin film application One of the most important methods for preparing thin films and coatings •Protection (corrosion, wear…) •Optical properties •Electronic properties •Magnetic properties •Decoration •New properties ...
Slide 1
... 3.2 Characteristic chemical groups help determine the properties of organic compounds ...
... 3.2 Characteristic chemical groups help determine the properties of organic compounds ...
b) Mole
... (49) From which part of the earth Aluminium is available in the form of oxide or sulphide ? (a) Atmosphere ...
... (49) From which part of the earth Aluminium is available in the form of oxide or sulphide ? (a) Atmosphere ...
BONUS: Which line in the above graph represents G for the reaction
... Which combination of pressure and temperature gives the highest yield of Z at equilibrium? (A) ...
... Which combination of pressure and temperature gives the highest yield of Z at equilibrium? (A) ...
enthalpy - winterk
... Give an example of an addition polymer and be able to explain how it forms. Name the two types of condensation polymers and give an example of each. Be sure to name the functional groups that must be present in the monomers of each type of condensation polymer. e) How are addition & condensation pol ...
... Give an example of an addition polymer and be able to explain how it forms. Name the two types of condensation polymers and give an example of each. Be sure to name the functional groups that must be present in the monomers of each type of condensation polymer. e) How are addition & condensation pol ...
Balancing Chemical Equations
... Chlorine is the most complex There are 3 Cl’s in the reactants and 2 Cl’s in the products The least common multiple between 3 and 2 is…6 In order to make 6 Cl’s on each side, put the coefficient 2 in front of the AlCl3 and the coefficient 3 in front of the CaCl2. ...
... Chlorine is the most complex There are 3 Cl’s in the reactants and 2 Cl’s in the products The least common multiple between 3 and 2 is…6 In order to make 6 Cl’s on each side, put the coefficient 2 in front of the AlCl3 and the coefficient 3 in front of the CaCl2. ...
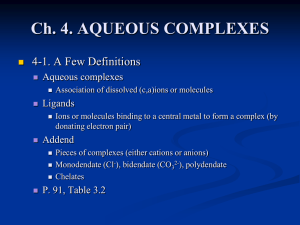
Ch. 3. KINETIC VS. EQUILIBRIUM MODELING
... Ions or molecules binding to a central metal to form a complex (by donating electron pair) ...
... Ions or molecules binding to a central metal to form a complex (by donating electron pair) ...
Topic 16 Test - A
... How many structural isomers, which are aldehydes, have the molecular formula C5H10O? A ...
... How many structural isomers, which are aldehydes, have the molecular formula C5H10O? A ...
Hydrocarbons
... oxidised to create carboxylic acids. We can use acidified dichromate or acidified permanganate as oxidising agents Elimination – As you might have guessed elimination reactions remove parts of the alcohol. In this case we remove the –OH group and one other hydrogen using concentrated sulfuric acid. ...
... oxidised to create carboxylic acids. We can use acidified dichromate or acidified permanganate as oxidising agents Elimination – As you might have guessed elimination reactions remove parts of the alcohol. In this case we remove the –OH group and one other hydrogen using concentrated sulfuric acid. ...
Spring 2015 CH 421 Name ________________________________________ 1. Consider the structures of vanillin and vanillyl alcohol.
... two mobile phases 1:1 hexanes:ethyl acetate and 4:1 hexanes:ethyl acetate. ...
... two mobile phases 1:1 hexanes:ethyl acetate and 4:1 hexanes:ethyl acetate. ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.