Document
... The empirical formula of a compound is CH2 Which molecular formula is correctly paired with a structural formula for this compound? ...
... The empirical formula of a compound is CH2 Which molecular formula is correctly paired with a structural formula for this compound? ...
lecture 1 - alcohols-ethers
... • Was once widely used as an anesthetic. • Highly flammable. • Presently used as a solvent. • Prepared by the dehydration of ethanol. ...
... • Was once widely used as an anesthetic. • Highly flammable. • Presently used as a solvent. • Prepared by the dehydration of ethanol. ...
Alcohols, phenols and ethers
... Thus, alcohols might be water-soluble, or not (depending on the length of the carbon chain). • We already saw that the boiling points of alkanes increase with increasing chain length. The same is true for alcohols. • Alcohols with more than one hydroxyl group (polyhydroxy alcohols) have higher boili ...
... Thus, alcohols might be water-soluble, or not (depending on the length of the carbon chain). • We already saw that the boiling points of alkanes increase with increasing chain length. The same is true for alcohols. • Alcohols with more than one hydroxyl group (polyhydroxy alcohols) have higher boili ...
Resolution of Diols via Catalytic Asymmetric Acetalization
... Theoretical conversion calculated from ee values of the product and the recovered starting material (see SI). bIsolated yield on a 0.5 mmol scale. Determined by HPLC analysis on a chiral stationary phase. dDetermined by GC analysis on a chiral stationary phase. eFor most minor diastereomers er >99.5 ...
... Theoretical conversion calculated from ee values of the product and the recovered starting material (see SI). bIsolated yield on a 0.5 mmol scale. Determined by HPLC analysis on a chiral stationary phase. dDetermined by GC analysis on a chiral stationary phase. eFor most minor diastereomers er >99.5 ...
PDF document
... The aim of this work is to develop a new kinetic spectrophotometric method for the determination of acetylsalicylic acid (ASA) in pharmaceutical formulations. In general, ASA analysis is not realised directly, and a previous quantitative hydrolysis in a basic medium is necessary, converting ASA to s ...
... The aim of this work is to develop a new kinetic spectrophotometric method for the determination of acetylsalicylic acid (ASA) in pharmaceutical formulations. In general, ASA analysis is not realised directly, and a previous quantitative hydrolysis in a basic medium is necessary, converting ASA to s ...
IOSR Journal of Applied Chemistry (IOSR-JAC) e-ISSN: 2278-5736.
... Heterocyclic compounds are widely distributed in nature and essential to much biochemical Process. These compounds are worth attention because of their biological activities and clinical usage Schiff base ligand forms a stable complex with different transition metal ions and has been the subject for ...
... Heterocyclic compounds are widely distributed in nature and essential to much biochemical Process. These compounds are worth attention because of their biological activities and clinical usage Schiff base ligand forms a stable complex with different transition metal ions and has been the subject for ...
Some Reactions Involving [W(N-2,6-Me C H )(OCMe
... mer-W(OCMe2CF3)3I3, the identities and connectivities of which were determined through X-ray crystallographic techniques.16 Complete structural characterization of these W(VI) compounds was not pursued. It is clear that ligand transfer from one metal to another and W-W bond cleavage to yield monomer ...
... mer-W(OCMe2CF3)3I3, the identities and connectivities of which were determined through X-ray crystallographic techniques.16 Complete structural characterization of these W(VI) compounds was not pursued. It is clear that ligand transfer from one metal to another and W-W bond cleavage to yield monomer ...
Tetrahedral, Highly Coordinatively Unsaturated 14e (Fe) and 15e
... Co) valence electrons. It is remarkable that the ethyl complexes 6 are thermally stable even at higher temperatures (70 °C (Co); 110 °C (Fe)), although they contain β-hydrogen atoms. The presence of a metal-carbon bond in 4-6 has been confirmed by protonation and hydrogenolysis, giving alkane (R-H) ...
... Co) valence electrons. It is remarkable that the ethyl complexes 6 are thermally stable even at higher temperatures (70 °C (Co); 110 °C (Fe)), although they contain β-hydrogen atoms. The presence of a metal-carbon bond in 4-6 has been confirmed by protonation and hydrogenolysis, giving alkane (R-H) ...
carbohydrates
... hydroxyl group (glycosidic hydroxyl). If its position is identical to the D-configuration of the determining carbon of the projected formula, it is called as –anomer, while in the case of identity with L-configuration, it is –anomer. Anomers are in equilibrium and they can be transformed freely to ...
... hydroxyl group (glycosidic hydroxyl). If its position is identical to the D-configuration of the determining carbon of the projected formula, it is called as –anomer, while in the case of identity with L-configuration, it is –anomer. Anomers are in equilibrium and they can be transformed freely to ...
2007 National Qualifying Exam – Chemistry
... of the molecule, except for the following: H2O is aqua, NH3 is ammine and CO is carbonyl. (iv) The prefixes mono-, di-, tri-, tetra-, penta-, hexa-, etc, are used to denote the number of simple (typically unidentate) ligands; bis-, tris-, tetrakis-, etc for more complicated (bidentate) ligands or on ...
... of the molecule, except for the following: H2O is aqua, NH3 is ammine and CO is carbonyl. (iv) The prefixes mono-, di-, tri-, tetra-, penta-, hexa-, etc, are used to denote the number of simple (typically unidentate) ligands; bis-, tris-, tetrakis-, etc for more complicated (bidentate) ligands or on ...
Review of selective separations of cobalt, uranium, zinc, nickel and
... Pb and Ni. Uranium feed solutions can contain significant amounts of Fe, Cu, Co and Ni. An important challenge in processing primary metal ores is the selective separation, recovery and disposition of the associated metals. These metals are often found in small quantities which compounds the problem ...
... Pb and Ni. Uranium feed solutions can contain significant amounts of Fe, Cu, Co and Ni. An important challenge in processing primary metal ores is the selective separation, recovery and disposition of the associated metals. These metals are often found in small quantities which compounds the problem ...
SOLUBILITY RULES FOR IONIC COMPOUNDS IN WATER
... (b) Calculate Kc for this reaction at 503 K, with proper units. (c) Calculate Kp for this reaction at 503 K, with proper units. 2. Ammonia decomposes according to the reaction: 2NH3 (g) ⇆ N2 (g) + 3H2 (g) A 2.00 liter tank is originally charged with 0.500 moles of ammonia, and at equilibrium it is f ...
... (b) Calculate Kc for this reaction at 503 K, with proper units. (c) Calculate Kp for this reaction at 503 K, with proper units. 2. Ammonia decomposes according to the reaction: 2NH3 (g) ⇆ N2 (g) + 3H2 (g) A 2.00 liter tank is originally charged with 0.500 moles of ammonia, and at equilibrium it is f ...
on nomenclature. compounds other than hydrocarbons%
... categories alkanes, alkenes, alkynes, arenes, cycloalkanes, and so on. But when the compound has more than one functional group it is not always obvious which is the parent function. For example, Compound 1 could be named as an alkene (because of the double-bond function) or as an alcohol (because o ...
... categories alkanes, alkenes, alkynes, arenes, cycloalkanes, and so on. But when the compound has more than one functional group it is not always obvious which is the parent function. For example, Compound 1 could be named as an alkene (because of the double-bond function) or as an alcohol (because o ...
Experiment 22
... the product of [H+] and [OH-] must be very small. In pure water, [H+] equals [OH-] equals 1 10-7 M. Although the product, [H+] [OH-] is small, that does not mean that both concentrations are necessarily small. If, for example, we dissolve HCl in water, the HCl in the solution will dissociate com ...
... the product of [H+] and [OH-] must be very small. In pure water, [H+] equals [OH-] equals 1 10-7 M. Although the product, [H+] [OH-] is small, that does not mean that both concentrations are necessarily small. If, for example, we dissolve HCl in water, the HCl in the solution will dissociate com ...
New Exp8
... Limitations of E1 Reaction: Acid-Catalyzed Dehydrations Competition can occur with SN1 reaction if reaction conditions are not ‘controlled’ (when protic solvents, non-basic nucleophiles are used). Mixtures of products form with the E1 reaction (also SN1). Unsymmetrical reagents and rearrangements po ...
... Limitations of E1 Reaction: Acid-Catalyzed Dehydrations Competition can occur with SN1 reaction if reaction conditions are not ‘controlled’ (when protic solvents, non-basic nucleophiles are used). Mixtures of products form with the E1 reaction (also SN1). Unsymmetrical reagents and rearrangements po ...
Chemistry 30 – Organic Chemistry
... Organic Chemistry – 15.2 – Polymers and the Petrochemical Industry • Ethene (ethylene) is required for the manufacture of many substances in Alberta’s petrochemical industry • Ethane, obtained from petroleum refining is “cracked” to produce ethene by catalytic cracking: Pt C2H6(g) CH2=CH2(g) + H2(g ...
... Organic Chemistry – 15.2 – Polymers and the Petrochemical Industry • Ethene (ethylene) is required for the manufacture of many substances in Alberta’s petrochemical industry • Ethane, obtained from petroleum refining is “cracked” to produce ethene by catalytic cracking: Pt C2H6(g) CH2=CH2(g) + H2(g ...
Document
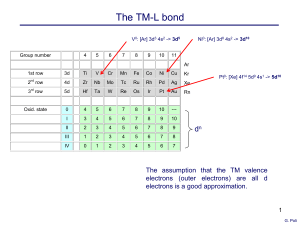
... The Molecular Orbital Theory In the MO approach, the molecular wavefunctions are a combination of the atomic orbitals, wherein the latter can physically overlap with each other. In particular, the orbitals of the ligands overlap with those of the metal to generate the coordination bonds. Since the ...
... The Molecular Orbital Theory In the MO approach, the molecular wavefunctions are a combination of the atomic orbitals, wherein the latter can physically overlap with each other. In particular, the orbitals of the ligands overlap with those of the metal to generate the coordination bonds. Since the ...
Thermodynamics
... During the polymerization of ethylene, one mole of ethylene breaks i.e. one C = C double bond breaks and the two CH2 ✂ groups are linked with C ✂ C single bonds thus forming three single bonds (two single bonds are formed when each CH2 ✂ group of ethylene links with one CH2 ✂ group of another ethyle ...
... During the polymerization of ethylene, one mole of ethylene breaks i.e. one C = C double bond breaks and the two CH2 ✂ groups are linked with C ✂ C single bonds thus forming three single bonds (two single bonds are formed when each CH2 ✂ group of ethylene links with one CH2 ✂ group of another ethyle ...
5 organic chemistry: functional groups
... can be difficult. Fortunately, there is another way to recognize oxidation–reduction reactions in organic chemistry: Oxidation occurs when hydrogen atoms are removed from a carbon atom or when an oxygen atom is added to a carbon atom. Reduction occurs when hydrogen atoms are added to a carbon atom o ...
... can be difficult. Fortunately, there is another way to recognize oxidation–reduction reactions in organic chemistry: Oxidation occurs when hydrogen atoms are removed from a carbon atom or when an oxygen atom is added to a carbon atom. Reduction occurs when hydrogen atoms are added to a carbon atom o ...
Palladium Complexes Containing Potentially
... No coalescence was observed for 3c up to 343 K, thus suggesting an activation energy of more than 65 kJ mol–1. Successful cleavage of the dimers was accomplished with methanolic HBr and produced the monometallic pyridylidene complexes 4a–c. An excess of HBr may be used without affecting the Pd–Cpyri ...
... No coalescence was observed for 3c up to 343 K, thus suggesting an activation energy of more than 65 kJ mol–1. Successful cleavage of the dimers was accomplished with methanolic HBr and produced the monometallic pyridylidene complexes 4a–c. An excess of HBr may be used without affecting the Pd–Cpyri ...
Chapter 15
... stead, a disordered array of polymeric chains, sheets, or three-dimensional units. Silica is relatively unreactive towards C1 2, H 2, acids, and most metals at 25°C or even at slightly elevated temperatures but is attacked by F2, aqueous HF, alkali hydroxides, and fused carbonates. Aqueous HF gives ...
... stead, a disordered array of polymeric chains, sheets, or three-dimensional units. Silica is relatively unreactive towards C1 2, H 2, acids, and most metals at 25°C or even at slightly elevated temperatures but is attacked by F2, aqueous HF, alkali hydroxides, and fused carbonates. Aqueous HF gives ...
Hydroformylation

Hydroformylation, also known as oxo synthesis or oxo process, is an important homogeneously catalyzed industrial process for the production of aldehydes from alkenes. This chemical reaction entails the addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon double bond. This process has undergone continuous growth since its invention in 1938: Production capacity reached 6.6×106 tons in 1995. It is important because the resulting aldehydes are easily converted into many secondary products. For example, the resulting aldehydes are hydrogenated to alcohols that are converted to plasticizers or detergents. Hydroformylation is also used in specialty chemicals, relevant to the organic synthesis of fragrances and natural products. The development of hydroformylation, which originated within the German coal-based industry, is considered one of the premier achievements of 20th-century industrial chemistry.The process typically entails treatment of an alkene with high pressures (between 10 to 100 atmospheres) of carbon monoxide and hydrogen at temperatures between 40 and 200 °C. Transition metal catalysts are required.