A Guide to Organic Molecules
... 11.1.1 Vapour pressure – the pressure generated by a liquid when it changes from the liquid state to a gas. 11.1.2 Volatility – is the ease with which a substance turns into vapour. It is directly related to the vapour pressure. 11.1.3 Viscosity – a measure of a substance’s resistance to flow – soli ...
... 11.1.1 Vapour pressure – the pressure generated by a liquid when it changes from the liquid state to a gas. 11.1.2 Volatility – is the ease with which a substance turns into vapour. It is directly related to the vapour pressure. 11.1.3 Viscosity – a measure of a substance’s resistance to flow – soli ...
CC 2 097-110..7686hdisk chapter .. Page97
... peroxides indicating that oxygen was mostly likely reduced to hydrogen peroxide in the course of bleaching the transient. UVVis traces (Fig. 1) showed formation of intense UV and visible bands on photolysis suggestive of efficient photoreaction. These new transient absorption bands for 3 and 4 were ...
... peroxides indicating that oxygen was mostly likely reduced to hydrogen peroxide in the course of bleaching the transient. UVVis traces (Fig. 1) showed formation of intense UV and visible bands on photolysis suggestive of efficient photoreaction. These new transient absorption bands for 3 and 4 were ...
Remodeling of the natural product fumagillol
... Further optimization using La(OTf)3 and Zn(OTf)2 catalysts was next pursued. The transformations were robust and did not require inert atmosphere, nor special precautions for anhydrous solvent. Other nonpolar solvents provided similar regioselectivity, though toluene proved to be optimal, in which c ...
... Further optimization using La(OTf)3 and Zn(OTf)2 catalysts was next pursued. The transformations were robust and did not require inert atmosphere, nor special precautions for anhydrous solvent. Other nonpolar solvents provided similar regioselectivity, though toluene proved to be optimal, in which c ...
Results
... Conformation D is sufficiently destabilized so only A, B and C are expected to show decarbonylation reaction The following 3 families of mechanisms may be anticipated A: Unimolecular Internal SN2 type reactionmechanism (SNi) with alactone formation B: Unimolecular Internal Addition/elimination mecha ...
... Conformation D is sufficiently destabilized so only A, B and C are expected to show decarbonylation reaction The following 3 families of mechanisms may be anticipated A: Unimolecular Internal SN2 type reactionmechanism (SNi) with alactone formation B: Unimolecular Internal Addition/elimination mecha ...
CHAPTER 12. DECODING ORGANIC STRUCTURES: THE
... many cases, but not all, may indicate a fragrant substance. Alcohols with fewer than six carbons are water soluble because of hydrogen bonding. For larger molecules, we need to examine both the carbon "backbone" and the functional groups, and to compare these with other molecules in attempting to pr ...
... many cases, but not all, may indicate a fragrant substance. Alcohols with fewer than six carbons are water soluble because of hydrogen bonding. For larger molecules, we need to examine both the carbon "backbone" and the functional groups, and to compare these with other molecules in attempting to pr ...
Practice Exam 2
... (5 pts.) Glycerol (1,2,3-propanetriol) has a boiling point of 290 °C, while that of 1pentanol boils at 138 °C. Discuss the factors that contribute to the observed boiling points of each of these molecules, and then explain why glycerol has a higher boiling point than that of 1-pentanol. ...
... (5 pts.) Glycerol (1,2,3-propanetriol) has a boiling point of 290 °C, while that of 1pentanol boils at 138 °C. Discuss the factors that contribute to the observed boiling points of each of these molecules, and then explain why glycerol has a higher boiling point than that of 1-pentanol. ...
Post Lab - University of Missouri
... In the following table, record your observations of the two alkene tests you performed in the appropriate column. In the “Conclusions” column, record whether or not your observations are consistent with the presence of an alkene. Alkene test ...
... In the following table, record your observations of the two alkene tests you performed in the appropriate column. In the “Conclusions” column, record whether or not your observations are consistent with the presence of an alkene. Alkene test ...
Carbon-Carbon Bond Formation by Reductive
... couplings, that of diphenylacetylene to yield exclusively (E,E)-1,2,3,4-tetraphenyl-l,3-buta diene and that of acetophenone to produce only racem/c-2,3-diphenyl-2,3-butanediol, is inter preted to conclude that the couplings proceed via two electron transfer pathways (TET) involving titanium (IV ) ...
... couplings, that of diphenylacetylene to yield exclusively (E,E)-1,2,3,4-tetraphenyl-l,3-buta diene and that of acetophenone to produce only racem/c-2,3-diphenyl-2,3-butanediol, is inter preted to conclude that the couplings proceed via two electron transfer pathways (TET) involving titanium (IV ) ...
Nomenclature of Carboxylic Acids 1. Parent alkane + the suffix
... corresponding carboxylic acid. The reaction is slower than the oxidation of alkenes, allowing disubstituted alkene carbons to be oxidized to the ketone, without significant over-oxidation. Aldehydes are smoothly oxidized to the corresponding carboxylic acid with either CrO3/H2SO4 or sodium dichromat ...
... corresponding carboxylic acid. The reaction is slower than the oxidation of alkenes, allowing disubstituted alkene carbons to be oxidized to the ketone, without significant over-oxidation. Aldehydes are smoothly oxidized to the corresponding carboxylic acid with either CrO3/H2SO4 or sodium dichromat ...
Chemistry in Action: Question paper - A
... (iii) State how barium sulfate can be used in medicine. Explain why this use is possible, given that solutions containing barium ions are poisonous. Use ............................................................................................................................ Explanation .......... ...
... (iii) State how barium sulfate can be used in medicine. Explain why this use is possible, given that solutions containing barium ions are poisonous. Use ............................................................................................................................ Explanation .......... ...
Reduction [H]
... Thioacetal (or thioketal) reduction or Mozingo reduction with Raney nickel and hydrogen is a classic method to prepare a methylene group from a carbonyl compound. Raney Nickel was developed in 1926 by American engineer Murray Raney. ( "Method of producing finely-divided nickel,"U.S. patent 1,628,190 ...
... Thioacetal (or thioketal) reduction or Mozingo reduction with Raney nickel and hydrogen is a classic method to prepare a methylene group from a carbonyl compound. Raney Nickel was developed in 1926 by American engineer Murray Raney. ( "Method of producing finely-divided nickel,"U.S. patent 1,628,190 ...
Ch14 Lecture
... • The “1” is usually omitted from the name. • The ring is then numbered to give the next substituent the lower number. ...
... • The “1” is usually omitted from the name. • The ring is then numbered to give the next substituent the lower number. ...
Ketones and Aldehydes Reading: Wade chapter 18, sections 18
... than alcohols. Nevertheless, aldehydes and ketones are able to engage alcohols and water in hydrogen bonding via the carbonyl oxygen lone-pairs, resulting in the solubility of polar substances like alcohols and water in lower aldehydes and ketones. ...
... than alcohols. Nevertheless, aldehydes and ketones are able to engage alcohols and water in hydrogen bonding via the carbonyl oxygen lone-pairs, resulting in the solubility of polar substances like alcohols and water in lower aldehydes and ketones. ...
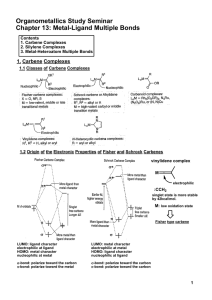
Metal-Ligand Multiple Bonds
... In all cases, these vinylidene complexes contain transition metals in relatively low oxidation state. Metal-acetylide complexes Vinylidene complex is thermodynamically unstable. ...
... In all cases, these vinylidene complexes contain transition metals in relatively low oxidation state. Metal-acetylide complexes Vinylidene complex is thermodynamically unstable. ...
Grignard Reaction - Synthesis of Substituted Benzoic Acids
... Analysis: Obtain a yield of your benzoic acid derivative. Measure the IR spectrum of the benzoic acid, submit a 1H NMR (in ~0.7 mL CDCl3) and an LC/MS (<1 mg in 1.5 mL MeOH). Submit your sample in a scintillation vial labeled with your name and structure to your TF, who will save your benzoic acid d ...
... Analysis: Obtain a yield of your benzoic acid derivative. Measure the IR spectrum of the benzoic acid, submit a 1H NMR (in ~0.7 mL CDCl3) and an LC/MS (<1 mg in 1.5 mL MeOH). Submit your sample in a scintillation vial labeled with your name and structure to your TF, who will save your benzoic acid d ...
Grignard Reaction - OpenBU
... Analysis: Obtain a yield of your benzoic acid derivative. Measure the IR spectrum of the benzoic acid, submit a 1H NMR (in ~0.7 mL CDCl3) and an LC/MS (<1 mg in 1.5 mL MeOH). Submit your sample in a scintillation vial labeled with your name and structure to your TF, who will save your benzoic acid d ...
... Analysis: Obtain a yield of your benzoic acid derivative. Measure the IR spectrum of the benzoic acid, submit a 1H NMR (in ~0.7 mL CDCl3) and an LC/MS (<1 mg in 1.5 mL MeOH). Submit your sample in a scintillation vial labeled with your name and structure to your TF, who will save your benzoic acid d ...
Grignard Reaction - OpenBU
... Analysis: Obtain a yield of your benzoic acid derivative. Measure the IR spectrum of the benzoic acid, submit a 1H NMR (in ~0.7 mL CDCl3) and an LC/MS (<1 mg in 1.5 mL MeOH). Submit your sample in a scintillation vial labeled with your name and structure to your TF, who will save your benzoic acid d ...
... Analysis: Obtain a yield of your benzoic acid derivative. Measure the IR spectrum of the benzoic acid, submit a 1H NMR (in ~0.7 mL CDCl3) and an LC/MS (<1 mg in 1.5 mL MeOH). Submit your sample in a scintillation vial labeled with your name and structure to your TF, who will save your benzoic acid d ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.
















![Reduction [H]](http://s1.studyres.com/store/data/007148356_1-25f5210a5c809c157bb6e0d32d66fd9d-300x300.png)