The Chemicals of Life Properties of Organic Compounds Organic
... It has a high electronegativity, so it pulls electrons away from the carbon atom It is important in carbohydrates, pyruvic acid (used in respiration), glycerol (a component of fats), and alcohols Alcohols are compounds that contain a hydroxyl group (OH-) attached to the carbon, and can easily dissol ...
... It has a high electronegativity, so it pulls electrons away from the carbon atom It is important in carbohydrates, pyruvic acid (used in respiration), glycerol (a component of fats), and alcohols Alcohols are compounds that contain a hydroxyl group (OH-) attached to the carbon, and can easily dissol ...
Regulations of the International Chemistry Olympiad (IChO)
... Electrophilic substitution: substitution on aromatic rings, influence of substituents on the reactivity and regioselectivity, electrophilic species; Elimination: E1 and E2 reactions at sp3 carbon centers, stereochemistry, acid-base catalysis, common leaving groups; Nucleophilic substitution: SN1 and ...
... Electrophilic substitution: substitution on aromatic rings, influence of substituents on the reactivity and regioselectivity, electrophilic species; Elimination: E1 and E2 reactions at sp3 carbon centers, stereochemistry, acid-base catalysis, common leaving groups; Nucleophilic substitution: SN1 and ...
CHM 3200 - Miami Dade College
... c. Illustrating methodologies for the synthesis of ketones (via oxidation of secondary alcohols). d. Identifying and illustrating reactions of aldehydes and ketones (nucleophilic addition reactions, hydration, acetal formation, Grignard reaction, conjugate addition, α-substitution reactions, nucleop ...
... c. Illustrating methodologies for the synthesis of ketones (via oxidation of secondary alcohols). d. Identifying and illustrating reactions of aldehydes and ketones (nucleophilic addition reactions, hydration, acetal formation, Grignard reaction, conjugate addition, α-substitution reactions, nucleop ...
File - Loreto Science
... • The Br+ species in order to gain the two electrons it needs to give it a stable octet of electrons attacks the C2H4 molecule • It forms a covalent bond with one of the carbon atoms • The other carbon atom is left with a positive charge and is now called a ...
... • The Br+ species in order to gain the two electrons it needs to give it a stable octet of electrons attacks the C2H4 molecule • It forms a covalent bond with one of the carbon atoms • The other carbon atom is left with a positive charge and is now called a ...
Recall
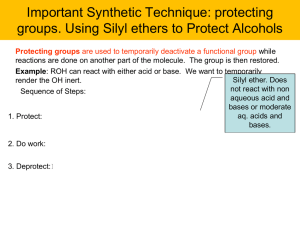
... reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily ...
... reactions are done on another part of the molecule. The group is then restored. Example: ROH can react with either acid or base. We want to temporarily ...
Bio 210 Cell Chemistry Lecture 3 Carbon Chemistry
... Isomers: compounds that have the same formula, but different structures and different properties. butane and isobutane C4H10 Types of isomers: Fig. 4.6 a. structural b. geometric c. enantiomers Structural isomers: vary in the arrangement of atoms, for example butane and isobutane. Geometric isomers: ...
... Isomers: compounds that have the same formula, but different structures and different properties. butane and isobutane C4H10 Types of isomers: Fig. 4.6 a. structural b. geometric c. enantiomers Structural isomers: vary in the arrangement of atoms, for example butane and isobutane. Geometric isomers: ...
Name Period_______________ Due
... Procedure: In this investigation, you will be using a 3-D chemical model kit to build different molecules. Look through your kit. The colored spheres represent different types of atoms. Notice that each sphere has a number of holes drilled into the sides. These represent the number of covalent bonds ...
... Procedure: In this investigation, you will be using a 3-D chemical model kit to build different molecules. Look through your kit. The colored spheres represent different types of atoms. Notice that each sphere has a number of holes drilled into the sides. These represent the number of covalent bonds ...
IUPAC nomenclature for organic chemistry
... • Name the groups attached to the longest carbon chain • Number the chain consecutively, starting at the end nearest a substituted group • Designate the location of each substituent group • Assemble the name by listing groups in alphabetical order and the main chain last ...
... • Name the groups attached to the longest carbon chain • Number the chain consecutively, starting at the end nearest a substituted group • Designate the location of each substituent group • Assemble the name by listing groups in alphabetical order and the main chain last ...
6.10 Acid-Catalyzed Hydration of Alkenes
... Hydroboration can be viewed as the addition of borane (BH3) to the double bond. But BH3 is not the reagent actually used. ...
... Hydroboration can be viewed as the addition of borane (BH3) to the double bond. But BH3 is not the reagent actually used. ...
06 MC /08 MC /08 NMR
... Rank the following in order of decreasing reactivity with bromine, Brz. ...
... Rank the following in order of decreasing reactivity with bromine, Brz. ...
name Page 1 of 6 Multiple Choice. Choose the best answer for the
... Which of the following statements concerning a carbocation is not true? (a) (b) (c) (d) (e) ...
... Which of the following statements concerning a carbocation is not true? (a) (b) (c) (d) (e) ...
Chapter15
... (3) Alkynes – triple bonds (4) Aromatic Hydrocarbons - rings Alkanes – CnH2n+2 Each carbon is sp3 hybridized ...
... (3) Alkynes – triple bonds (4) Aromatic Hydrocarbons - rings Alkanes – CnH2n+2 Each carbon is sp3 hybridized ...
Structure of Organic Compounds Infra
... From the molecular formula we can calculate what is known as Units of unsaturation Double bond equivalents Degree of unsaturation Index of hydrogen deficiency These terms are all synonymous. A double bond or a ring counts as a double bond equivalent. We can calculate the number of double bond ...
... From the molecular formula we can calculate what is known as Units of unsaturation Double bond equivalents Degree of unsaturation Index of hydrogen deficiency These terms are all synonymous. A double bond or a ring counts as a double bond equivalent. We can calculate the number of double bond ...
• Pergamon
... MetaHoporphyrins have been used extensively as models for studying cytochrome P-450 monooxygenase activity.! Although these catalysts are known to carry out many useful reactions such as alkene epoxidation and hydrocarbon oxidation, their utility in organic synthesis has been limited. Recently we re ...
... MetaHoporphyrins have been used extensively as models for studying cytochrome P-450 monooxygenase activity.! Although these catalysts are known to carry out many useful reactions such as alkene epoxidation and hydrocarbon oxidation, their utility in organic synthesis has been limited. Recently we re ...
naming using more functional groups
... • analysis of this showed that all six C-C bonds had identical lengths (140 pm) – this allows the molecule to be symmetrical instead of the different lengths associated with singe (154 pm) and double (134 pm) ...
... • analysis of this showed that all six C-C bonds had identical lengths (140 pm) – this allows the molecule to be symmetrical instead of the different lengths associated with singe (154 pm) and double (134 pm) ...
Unit 11 – Alcohols, Phenols and Ethers
... A. Due to highly activating effect of –OH group, the polarization of Br2 molecule takes place even in the absence of Lewis acid like FeBr3. 20. Preparation of ethers by dehydration of alcohols is suitable from primary alcohols only. A. Reaction follows SN2 mechanism with primary alcohol and ether is ...
... A. Due to highly activating effect of –OH group, the polarization of Br2 molecule takes place even in the absence of Lewis acid like FeBr3. 20. Preparation of ethers by dehydration of alcohols is suitable from primary alcohols only. A. Reaction follows SN2 mechanism with primary alcohol and ether is ...
BIOB111 - Tutorial activities for session 8
... The three functional groups of hydrocarbons that contain only hydrogen and carbon atoms are alkane, alkene and alkyne. Through the use of chemical reactions, it is possible for an alkane to become an alkyne and visa versa. How is an alkyne converted to an alkane? ...
... The three functional groups of hydrocarbons that contain only hydrogen and carbon atoms are alkane, alkene and alkyne. Through the use of chemical reactions, it is possible for an alkane to become an alkyne and visa versa. How is an alkyne converted to an alkane? ...
Alkene

In organic chemistry, an alkene is an unsaturated hydrocarbon that contains at least one carbon–carbon double bond. Alkene, olefin, and olefine are used often interchangeably (see nomenclature section below). Acyclic alkenes, with only one double bond and no other functional groups, known as mono-enes, form a homologous series of hydrocarbons with the general formula CnH2n. Alkenes have two hydrogen atoms less than the corresponding alkane (with the same number of carbon atoms). The simplest alkene, ethylene (C2H4), which has the International Union of Pure and Applied Chemistry (IUPAC) name ethene is the organic compound produced on the largest scale industrially. Aromatic compounds are often drawn as cyclic alkenes, but their structure and properties are different and they are not considered to be alkenes.