07.Chapter7.Alcohols and Related
... Convert the hydroxyl group to a good leaving group!!!! Since hydrogen chloride and sulfur dioxide are gases and evolve from the product, we don’t need to separate the desired alkyl halide from the mixture!!!! ...
... Convert the hydroxyl group to a good leaving group!!!! Since hydrogen chloride and sulfur dioxide are gases and evolve from the product, we don’t need to separate the desired alkyl halide from the mixture!!!! ...
Lecture 28 - The Cook Group @ NDSU
... The substitution of an alcohol functional group with a halogen can be carried out on tertiary alcohols with mineral acids. This reaction only works with 3° alcohols as the mechanism involves the loss of water to form a carbocation and subsequent addition of halide. H OH ...
... The substitution of an alcohol functional group with a halogen can be carried out on tertiary alcohols with mineral acids. This reaction only works with 3° alcohols as the mechanism involves the loss of water to form a carbocation and subsequent addition of halide. H OH ...
These two compounds are structural isomers, which would have the
... November 27, 2014 Naming Esters -the name of the ester is derived from the parent alcohol and acid -find the functional group and draw a line through the middle of the functional group ...
... November 27, 2014 Naming Esters -the name of the ester is derived from the parent alcohol and acid -find the functional group and draw a line through the middle of the functional group ...
Things to remember in the last hour before the
... Classification of alcohols and haloalkanes as primary, secondary or tertiary – look how many C atoms are attached to the C atom that is C-OH or C-X. ...
... Classification of alcohols and haloalkanes as primary, secondary or tertiary – look how many C atoms are attached to the C atom that is C-OH or C-X. ...
Summary of Organic Compounds -Functional Groups and Reactions
... Summary of Organic Compounds- Functional Groups and Reactions ...
... Summary of Organic Compounds- Functional Groups and Reactions ...
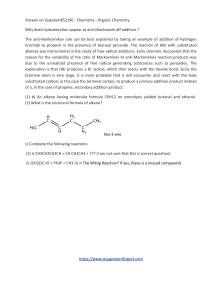
Answer on Question#52196 - Chemistry
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
... alkenes was instrumental in the study of free-radical additions. Early chemists discovered that the reason for the variability of the ratio of Markovnikov to anti-Markovnikov reaction products was due to the unrealized presence of free radical generating substances such as peroxides. The explanation ...
Preparation of alkyl halides There are lots of ways to make alkyl
... to use one of the special halogenating agents shown below: ...
... to use one of the special halogenating agents shown below: ...
HYDROCARBON DERIVATIVES Hydrocarbons are compounds
... replaced with a nonhydrocarbon element or group of elements (functional group) Ex. CH3CH2Cl What is a 'functional' group? Organic Halides an organic molecule in which one or more of the hydrogens have been replaced with a Group 17 (halogens) atom. Naming Organic halides are named using the same ...
... replaced with a nonhydrocarbon element or group of elements (functional group) Ex. CH3CH2Cl What is a 'functional' group? Organic Halides an organic molecule in which one or more of the hydrogens have been replaced with a Group 17 (halogens) atom. Naming Organic halides are named using the same ...
haloalkanes (halogenoalkanes)
... process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 is favoured for tertiary haloalkanes where there is steric hindrance to the attack and a more ...
... process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 is favoured for tertiary haloalkanes where there is steric hindrance to the attack and a more ...
halogenoalkanes (haloalkanes)
... This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 ...
... This form of nucleophilic substitution discussed so far is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 ...
Organic Synthesis
... which have at least one lone pair of electrons that they can donate and form dative bonds. ...
... which have at least one lone pair of electrons that they can donate and form dative bonds. ...
aminoalkanes (or amines)
... AMINOALKANES (OR AMINES) Introduction: The IUPAC names for amines are aminoalkanes or alkanamines. E.g.: CH3CH2NH2 Ethylamine – amine –common name Aminoethane – aminoalkane – systematic name Ethanamine – alkanamine – systematic name Mines all contain a nitrogen atom in the organic molecule. Th ...
... AMINOALKANES (OR AMINES) Introduction: The IUPAC names for amines are aminoalkanes or alkanamines. E.g.: CH3CH2NH2 Ethylamine – amine –common name Aminoethane – aminoalkane – systematic name Ethanamine – alkanamine – systematic name Mines all contain a nitrogen atom in the organic molecule. Th ...
Chemistry - Choithram School
... ii) Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions. iii)Reaction of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in presence of alcoholic KOH , alkenes are the major products. iv)Chloroform is stored in closed dark coloured b ...
... ii) Haloarenes are much less reactive than haloalkanes towards nucleophilic substitution reactions. iii)Reaction of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in presence of alcoholic KOH , alkenes are the major products. iv)Chloroform is stored in closed dark coloured b ...
Slides for Chapter 1-4 - Department of Chemistry and Physics
... Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination ...
... Nucleophiles will replace the halide in C-X bonds of many alkyl halides(reaction as Lewis base) Nucleophiles that are Brønsted bases produce elimination ...
Classification of Halogen Derivatives
... kCN is predominantly ionic and provides cyanide ions in solution, which is ambident nucleophile and bind with carbon side to form as the major product, while AgCN is covalent and form isocyanide as the major product. Like KCN, KNO2 form R-ONO while AgNO2 produces R-NO2 as product. Vinyl chloride is ...
... kCN is predominantly ionic and provides cyanide ions in solution, which is ambident nucleophile and bind with carbon side to form as the major product, while AgCN is covalent and form isocyanide as the major product. Like KCN, KNO2 form R-ONO while AgNO2 produces R-NO2 as product. Vinyl chloride is ...
Organometallic Reagents: Sources of Nucleophilic Carbon for
... Grignard reagents, RMgX, can be formed from primary, secondary, and tertiary haloalkane, as well as from haloalkenes and halobenzenes. Grignard reagents are very sensitive to moisture and air and are formed in solution and ...
... Grignard reagents, RMgX, can be formed from primary, secondary, and tertiary haloalkane, as well as from haloalkenes and halobenzenes. Grignard reagents are very sensitive to moisture and air and are formed in solution and ...
Rates of Hydrolysis of Some Halogeno-compounds
... 1. the carbon-halogen bond is strengthened by its partial -bond character. The breakage of the bond requires a larger amount of energy and so the substitution reaction becomes more difficult, and 2. the polarity of carbon-halogen bond is decreased, making the C atom much less susceptible to nucleop ...
... 1. the carbon-halogen bond is strengthened by its partial -bond character. The breakage of the bond requires a larger amount of energy and so the substitution reaction becomes more difficult, and 2. the polarity of carbon-halogen bond is decreased, making the C atom much less susceptible to nucleop ...
haloalkanes - Knockhardy
... This form of nucleophilic substitution is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 is favoured for t ...
... This form of nucleophilic substitution is known as SN2; it is a bimolecular process. An alternative method involves the initial breaking of the C-X bond to form a carbocation, or carbonium ion, (a unimolecular process - SN1 mechanism), which is then attacked by the nucleophile. SN1 is favoured for t ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.