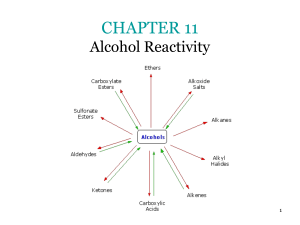
Carbonyl The carbonyl function, C=O, exists in a number of organic
... The stereochemistry of Wittig reactions is very interesting, and the detailed mechanisms involved are somewhat cloudy. As shown below the reagents add in a fast step to form an eclipsed betaine (or goes directly to the oxaphosphetane). The oxaphosphetane breaks down by syn elimination to give the tr ...
... The stereochemistry of Wittig reactions is very interesting, and the detailed mechanisms involved are somewhat cloudy. As shown below the reagents add in a fast step to form an eclipsed betaine (or goes directly to the oxaphosphetane). The oxaphosphetane breaks down by syn elimination to give the tr ...
Unit 13 Organic Chem R
... A molecule consisting of two alkyl groups on either side of an oxygen atom. The dehydration synthesis of an ether by reacting two molecules of primary alcohol. The anaerobic digestion of simple sugars by yeast to produce ethanol and carbon dioxide. A hydrocarbon that has had one or more halogen atom ...
... A molecule consisting of two alkyl groups on either side of an oxygen atom. The dehydration synthesis of an ether by reacting two molecules of primary alcohol. The anaerobic digestion of simple sugars by yeast to produce ethanol and carbon dioxide. A hydrocarbon that has had one or more halogen atom ...
Electophilic Aromatic Substituion
... Reaction with a mixture of sulfuric acid and SO3 (“Fuming H2SO4) Reactive species is sulfur trioxide or its conjugate acid ...
... Reaction with a mixture of sulfuric acid and SO3 (“Fuming H2SO4) Reactive species is sulfur trioxide or its conjugate acid ...
Electophilic Aromatic Substituion
... Reaction with a mixture of sulfuric acid and SO3 (“Fuming H2SO4) Reactive species is sulfur trioxide or its conjugate acid ...
... Reaction with a mixture of sulfuric acid and SO3 (“Fuming H2SO4) Reactive species is sulfur trioxide or its conjugate acid ...
Unit 13 Organic Chem R
... A molecule consisting of two alkyl groups on either side of an oxygen atom. The dehydration synthesis of an ether by reacting two molecules of primary alcohol. The anaerobic digestion of simple sugars by yeast to produce ethanol and carbon dioxide. A hydrocarbon that has had one or more halogen atom ...
... A molecule consisting of two alkyl groups on either side of an oxygen atom. The dehydration synthesis of an ether by reacting two molecules of primary alcohol. The anaerobic digestion of simple sugars by yeast to produce ethanol and carbon dioxide. A hydrocarbon that has had one or more halogen atom ...
Document
... Example 9.3 Molecular Formulas of Hydrocarbons What is the molecular formula for 1-octene? (The “1-” is a part of a systematic name that indicates the location of the double bond; it follows the first carbon atom of the chain.) ...
... Example 9.3 Molecular Formulas of Hydrocarbons What is the molecular formula for 1-octene? (The “1-” is a part of a systematic name that indicates the location of the double bond; it follows the first carbon atom of the chain.) ...
Name (Last, First)
... Make models to show the reaction between propanoic acid (CH 3CH2COOH) and NaOH (an inorganic base) . Write an equation for the reaction of propanoic acid (CH3CH2COOH) with NaOH (an inorganic base) using structural formulas. Name the products. ...
... Make models to show the reaction between propanoic acid (CH 3CH2COOH) and NaOH (an inorganic base) . Write an equation for the reaction of propanoic acid (CH3CH2COOH) with NaOH (an inorganic base) using structural formulas. Name the products. ...
Unit 13 Organic Chem AE
... A molecule consisting of two alkyl groups on either side of an oxygen atom. The dehydration synthesis of an ether by reacting two molecules of primary alcohol. The anaerobic digestion of simple sugars by yeast to produce ethanol and carbon dioxide. A hydrocarbon that has had one or more halogen atom ...
... A molecule consisting of two alkyl groups on either side of an oxygen atom. The dehydration synthesis of an ether by reacting two molecules of primary alcohol. The anaerobic digestion of simple sugars by yeast to produce ethanol and carbon dioxide. A hydrocarbon that has had one or more halogen atom ...
Naming Alkanes Handout.key
... After this lesson you should be able to Name alkanes using IUPAC rules ...
... After this lesson you should be able to Name alkanes using IUPAC rules ...
Fisher Esterification - OpenBU
... reverse the order of this addition. Always add sulfuric acid to the solvent mixture to avoid splattering!) Secure the screw cap firmly over the top of the vessel (make sure your stirrer is inside!) and place your sample in the microwave rack – be sure to note your position. Your TF will then heat yo ...
... reverse the order of this addition. Always add sulfuric acid to the solvent mixture to avoid splattering!) Secure the screw cap firmly over the top of the vessel (make sure your stirrer is inside!) and place your sample in the microwave rack – be sure to note your position. Your TF will then heat yo ...
Grignard Reactions - faculty at Chemeketa
... RMgX The Grignard reagent is one of the most versatile and widely used reagents in organic chemistry. We will consider only its reactions with aldehydes and ketones at this time. Grignards react with aldehydes and ketones to give intermediate products that form alcohols when hydrolyzed. With formald ...
... RMgX The Grignard reagent is one of the most versatile and widely used reagents in organic chemistry. We will consider only its reactions with aldehydes and ketones at this time. Grignards react with aldehydes and ketones to give intermediate products that form alcohols when hydrolyzed. With formald ...
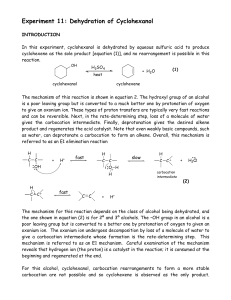
Dehydration of Cyclohexanol
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
... carbocations derived from certain 2°alcohols may undergo rearrangement to form more stable carbocations. This can result in the formation of rearranged isomeric alkenes. Both 2° and 3° alcohols primarily undergo the E1 reaction under these conditions, whereas for 1° alcohols and methyl alcohol, symm ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... a) Explain the octant rule with a suitable example. b) The cotton effect curves of (+)-3-methylcyclopentanone in methanol shows only a 24% reaction upon addition of acid a) Compare the ipso-attack in aromatic electrophilic substitution with the SNAr mechanism. b) ‘The reaction between ethylene oxide ...
... a) Explain the octant rule with a suitable example. b) The cotton effect curves of (+)-3-methylcyclopentanone in methanol shows only a 24% reaction upon addition of acid a) Compare the ipso-attack in aromatic electrophilic substitution with the SNAr mechanism. b) ‘The reaction between ethylene oxide ...
Ch 8 Lecture 1
... e) Organic molecules with polar functional groups (-OH, -NH2, -CO2H) have much higher water solubilities 2) The longer the alkyl group, the less water soluble the alcohol (more soluble in hydrocarbons—like dissolves like) 3) MeOH and EtOH are very similar to water as solvents: many salts will dissol ...
... e) Organic molecules with polar functional groups (-OH, -NH2, -CO2H) have much higher water solubilities 2) The longer the alkyl group, the less water soluble the alcohol (more soluble in hydrocarbons—like dissolves like) 3) MeOH and EtOH are very similar to water as solvents: many salts will dissol ...
Geoffrey Wilkinson - Nobel Lecture
... The synthesis of stable metal alkyls, and indeed, the nature of the transition metal to carbon bond, is a problem that has been with us for a long time. The first attempts to make such compounds were shortly after Frankland’s epoch-making discovery of diethylzinc. Thus in 1859 Buckton wrote, “a rich ...
... The synthesis of stable metal alkyls, and indeed, the nature of the transition metal to carbon bond, is a problem that has been with us for a long time. The first attempts to make such compounds were shortly after Frankland’s epoch-making discovery of diethylzinc. Thus in 1859 Buckton wrote, “a rich ...
blank lecture 11
... • Because of the polarity of the C=O group, these groups can interact, but the attraction is not as strong as hydrogen bonding. • This makes the boiling point of aldehydes and ketones ___________ than alkanes, but _________ than alcohols. ...
... • Because of the polarity of the C=O group, these groups can interact, but the attraction is not as strong as hydrogen bonding. • This makes the boiling point of aldehydes and ketones ___________ than alkanes, but _________ than alcohols. ...
Introduction to Oil Chemistry and Transesterification
... These kinks cause the molecules to resist solidification and ...
... These kinks cause the molecules to resist solidification and ...
Nucleophilic Addition: The Grignard reagent
... you can add a small iodine crystal to the reaction. Once the reaction has started and a brownish gray suspension has begun to form, slowly add the remaining bromobenzene– ether solution over fifteen minutes. It is important that the reaction stays warm. The exothermic reaction should keep the reacti ...
... you can add a small iodine crystal to the reaction. Once the reaction has started and a brownish gray suspension has begun to form, slowly add the remaining bromobenzene– ether solution over fifteen minutes. It is important that the reaction stays warm. The exothermic reaction should keep the reacti ...
Glossary of Key Terms in Chapter Two
... acyl group (15.3) the functional group found in the carboxylic acid derivatives that contains the carbonyl group attached to one alkyl or aryl group. alkaloid (15.2) a class of naturally occurring compounds that contain one or more nitrogen heterocyclic rings; many of the alkaloids have medicinal an ...
... acyl group (15.3) the functional group found in the carboxylic acid derivatives that contains the carbonyl group attached to one alkyl or aryl group. alkaloid (15.2) a class of naturally occurring compounds that contain one or more nitrogen heterocyclic rings; many of the alkaloids have medicinal an ...
a. b. c. d.
... substances. One is 2‐phenylethanol. Another is acetophenone (C6H5C(=O)CH3) and the third is phenylacetic acid (C6H5CH2COOH). Smell could help with the identity, but the worker has a cold, so samples of each of the three bottles are taken and H‐NMR, C‐NMR, and FTIR spectra of each bottle are made. ...
... substances. One is 2‐phenylethanol. Another is acetophenone (C6H5C(=O)CH3) and the third is phenylacetic acid (C6H5CH2COOH). Smell could help with the identity, but the worker has a cold, so samples of each of the three bottles are taken and H‐NMR, C‐NMR, and FTIR spectra of each bottle are made. ...
Sodium is an abundant metallic element with atomic number as 11
... -The reaction of sodium with alcohols is similar to the reaction of sodium with water, but slower. There are two general reactions with organic halides. One of them requires the condensation of two organic compounds, which form halogens when those are eliminated. The second type of reaction includes ...
... -The reaction of sodium with alcohols is similar to the reaction of sodium with water, but slower. There are two general reactions with organic halides. One of them requires the condensation of two organic compounds, which form halogens when those are eliminated. The second type of reaction includes ...
Haloalkane

The haloalkanes (also known, as halogenoalkanes or alkyl halides) are a group of chemical compounds derived from alkanes containing one or more halogens. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially and, consequently, are known under many chemical and commercial names. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes which contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula ″RX″ where R is an alkyl or substituted alkyl group and X is a halogen (F, Cl, Br, I).Haloalkanes have been known for centuries. Chloroethane was produced synthetically in the 15th century. The systematic synthesis of such compounds developed in the 19th century in step with the development of organic chemistry and the understanding of the structure of alkanes. Methods were developed for the selective formation of C-halogen bonds. Especially versatile methods included the addition of halogens to alkenes, hydrohalogenation of alkenes, and the conversion of alcohols to alkyl halides. These methods are so reliable and so easily implemented that haloalkanes became cheaply available for use in industrial chemistry because the halide could be further replaced by other functional groups.While most haloalkanes are human-produced, non-artificial-source haloalkanes do occur on Earth, mostly through enzyme-mediated synthesis by bacteria, fungi, and especially sea macroalgae (seaweeds). More than 1600 halogenated organics have been identified, with bromoalkanes being the most common haloalkanes. Brominated organics in biology range from biologically produced methyl bromide to non-alkane aromatics and unsaturates (indoles, terpenes, acetogenins, and phenols). Halogenated alkanes in land plants are more rare, but do occur, as for example the fluoroacetate produced as a toxin by at least 40 species of known plants. Specific dehalogenase enzymes in bacteria which remove halogens from haloalkanes, are also known.