Chem A Week 2 Matter Notes
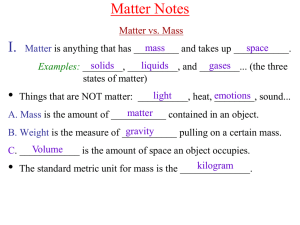
... A.Solids– are ____________ matter They have a definite shape and volume matter B. Liquids – are ___________ They take the shape of their container, volume but still have a definite ________________. ...
... A.Solids– are ____________ matter They have a definite shape and volume matter B. Liquids – are ___________ They take the shape of their container, volume but still have a definite ________________. ...
Leaving Certificate Chemistry
... Science education provides a means by which learners can interact with the world around them and understand how scientific concepts can be used to make sense of the physical world. As learners’ scientific literacy grows they will be able to make sense of the various ways in which scientific knowledg ...
... Science education provides a means by which learners can interact with the world around them and understand how scientific concepts can be used to make sense of the physical world. As learners’ scientific literacy grows they will be able to make sense of the various ways in which scientific knowledg ...
Classifying Reactions: A good summary
... will attract SO42- ions. Nevertheless, SO42- can't further oxidize (full of oxygen and no more unshared pair of electrons possible for further oxidation). As the result, you should use the other side of the hydrolysis in Trick #1: instead of OH-, put H+; instead of H2, put O2. This makes sense beca ...
... will attract SO42- ions. Nevertheless, SO42- can't further oxidize (full of oxygen and no more unshared pair of electrons possible for further oxidation). As the result, you should use the other side of the hydrolysis in Trick #1: instead of OH-, put H+; instead of H2, put O2. This makes sense beca ...
advanced placement chemistry alamo heights high school scope
... Activity: Effect on biological systems [CR4] Students examine a demonstration size model of DNA or an alpha helix, and use their fingers to identify which atoms / base pairs are particularly involved in ...
... Activity: Effect on biological systems [CR4] Students examine a demonstration size model of DNA or an alpha helix, and use their fingers to identify which atoms / base pairs are particularly involved in ...
Lecture 2 - Columbia University
... (2) Understand the atomic and molecular interpretation of elements, compounds and mixtures Element: a molecule that contains only one type of atom. Examples: Hydrogen molecules (H2), Oxygen molecules (O2), Ozone (O3), buckyballs (C60), Diamond (Cn), Graphite (Cn), Compound: a molecule that contains ...
... (2) Understand the atomic and molecular interpretation of elements, compounds and mixtures Element: a molecule that contains only one type of atom. Examples: Hydrogen molecules (H2), Oxygen molecules (O2), Ozone (O3), buckyballs (C60), Diamond (Cn), Graphite (Cn), Compound: a molecule that contains ...
Click here to Ch 06.2 Covalent Bonding_Lewis Structures
... • Exceptions to the octet rule include those for atoms that cannot fit eight electrons, and for those that can fit more than eight electrons, into their outermost orbital. • Hydrogen forms bonds in which it is surrounded by only two electrons. • Boron has just three valence electrons, so it tends to ...
... • Exceptions to the octet rule include those for atoms that cannot fit eight electrons, and for those that can fit more than eight electrons, into their outermost orbital. • Hydrogen forms bonds in which it is surrounded by only two electrons. • Boron has just three valence electrons, so it tends to ...
Structure of Molecules and Compounds | Principles of Biology from
... covalent molecules are attracted to one another. A hydrogen bond occurs when molecules that have hydrogen covalently bonded to oxygen, nitrogen or fluorine interact. For example, water is held together by hydrogen bonds that occur between the oxygen of one water molecule and a hydrogen on another (F ...
... covalent molecules are attracted to one another. A hydrogen bond occurs when molecules that have hydrogen covalently bonded to oxygen, nitrogen or fluorine interact. For example, water is held together by hydrogen bonds that occur between the oxygen of one water molecule and a hydrogen on another (F ...
Grade 9 Academic Science
... 1. What are the signs of physical change? Chemical change? How are they different? 2. What do atoms do to become stable? What does a metal like lithium or calcium do? What about a non-metal like sulfur or iodine? 3. How do atoms become ions? Do they lose or gain protons, electrons, and/or neutrons? ...
... 1. What are the signs of physical change? Chemical change? How are they different? 2. What do atoms do to become stable? What does a metal like lithium or calcium do? What about a non-metal like sulfur or iodine? 3. How do atoms become ions? Do they lose or gain protons, electrons, and/or neutrons? ...
BSC with Chemistry CBCS Syllabus 2016-17
... Ionic Bonding: General characteristics of ionic bonding. Energy considerations in ionic bonding, lattice energy and solvation energy and their importance in the context of stability and solubility of ionic compounds. Statement of Born-Landé equation for calculation of lattice energy, Born-Haber cycl ...
... Ionic Bonding: General characteristics of ionic bonding. Energy considerations in ionic bonding, lattice energy and solvation energy and their importance in the context of stability and solubility of ionic compounds. Statement of Born-Landé equation for calculation of lattice energy, Born-Haber cycl ...
1. Natures Chemistry Unit Questions
... (a) Draw a structural formula for the product formed when propanone is used instead of ethanal in this type of reaction. (1) (b) Name an aldehyde that would not take part in an aldol condensation. (1) (c) Apart from the structure of the reactants, suggest what is unusual about applying the term “con ...
... (a) Draw a structural formula for the product formed when propanone is used instead of ethanal in this type of reaction. (1) (b) Name an aldehyde that would not take part in an aldol condensation. (1) (c) Apart from the structure of the reactants, suggest what is unusual about applying the term “con ...
Types of Reactions and Solution Chemistry
... Acids and bases are important compounds. We have talked about how to name acids specifically the binary halogen acids (HF, HCl, HBr, and HI as being names hydrofluoric acid, hydrochloric acid, hydrobromic acid, and hydroiodic acid) and the oxoacids (H2SO4, H2CO3, HNO3, and HNO2 for example: sulfuri ...
... Acids and bases are important compounds. We have talked about how to name acids specifically the binary halogen acids (HF, HCl, HBr, and HI as being names hydrofluoric acid, hydrochloric acid, hydrobromic acid, and hydroiodic acid) and the oxoacids (H2SO4, H2CO3, HNO3, and HNO2 for example: sulfuri ...
Quantum Chemistry Predicts Multiply Bonded Diuranium
... formed between the uranium atoms as in the corresponding dichromium compound. This bond can be described with 10 active orbitals (σ, σ*, π, π*, δ, and δ*). In practice, 13 active orbitals were used in order to assure that no other bonding types would occur. They turned out have have very small occup ...
... formed between the uranium atoms as in the corresponding dichromium compound. This bond can be described with 10 active orbitals (σ, σ*, π, π*, δ, and δ*). In practice, 13 active orbitals were used in order to assure that no other bonding types would occur. They turned out have have very small occup ...
Science
... Chemistry should explore the composition of matter through its properties, its atomic structure, and the manner in which it bonds and reacts with other substances. Students should be expected to use suitable mathematics and collect and analyze data. Instruction and assessment should include both app ...
... Chemistry should explore the composition of matter through its properties, its atomic structure, and the manner in which it bonds and reacts with other substances. Students should be expected to use suitable mathematics and collect and analyze data. Instruction and assessment should include both app ...
Ionic Bonding
... helium). These noble gas structures are thought of as being in some way a "desirable" thing for an atom to have. You may well have been left with the strong impression that when other atoms react, they try to organize things such that their outer levels are either completely full or completely empty ...
... helium). These noble gas structures are thought of as being in some way a "desirable" thing for an atom to have. You may well have been left with the strong impression that when other atoms react, they try to organize things such that their outer levels are either completely full or completely empty ...
Volatile Organic Compounds
... Volatile Organic Compounds (VOC's) are organic (carbon-containing) chemicals that have a high vapours pressure and easily form vapours at normal temperature and pressure. VOC’s include trichloroethylene, benzene, toluene, styrene, acetone, ethyl benzene, mixed xylenes methyl ethyl ketone, alcohols, ...
... Volatile Organic Compounds (VOC's) are organic (carbon-containing) chemicals that have a high vapours pressure and easily form vapours at normal temperature and pressure. VOC’s include trichloroethylene, benzene, toluene, styrene, acetone, ethyl benzene, mixed xylenes methyl ethyl ketone, alcohols, ...
01.CN_Other pages/p1-9
... (b) (i) Which particle(s) is / are the ions? Hint 2 (ii) What is the relationship between P and Q? (iii) Do particles of P and Q have the same chemical properties? Explain your answer. (c) (i) Suggest a term to indicate the relationship between S and T. (ii) Explain why S and T have the same chemica ...
... (b) (i) Which particle(s) is / are the ions? Hint 2 (ii) What is the relationship between P and Q? (iii) Do particles of P and Q have the same chemical properties? Explain your answer. (c) (i) Suggest a term to indicate the relationship between S and T. (ii) Explain why S and T have the same chemica ...
1A - The changing atom History of the atom • The model of the atom
... 6) Gp 7 (Halogens) = -1 (except with oxygen) 7) O = -2 except in peroxides (H2O2, O = -1) Examples: Compounds CO2 ...
... 6) Gp 7 (Halogens) = -1 (except with oxygen) 7) O = -2 except in peroxides (H2O2, O = -1) Examples: Compounds CO2 ...
WELCOME TO AP CHEMISTRY
... (a) When a 0.2800 gram sample of this limestone was decomposed by heating, 0.00308 moles of CO2 were evolved. How many grams of CO2 were produced? ...
... (a) When a 0.2800 gram sample of this limestone was decomposed by heating, 0.00308 moles of CO2 were evolved. How many grams of CO2 were produced? ...
Inorganic chemistry

Inorganic chemistry deals with the synthesis and behavior of inorganic and organometallic compounds. This field covers all chemical compounds except the myriad organic compounds (carbon based compounds, usually containing C-H bonds), which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, and there is much overlap, most importantly in the sub-discipline of organometallic chemistry. It has applications in every aspect of the chemical industry–including catalysis, materials science, pigments, surfactants, coatings, medicine, fuel, and agriculture.























