File
... Directions: Each blank can be completed with a term, short phrase, or number. It is possible to __1__ the products of some chemical ...
... Directions: Each blank can be completed with a term, short phrase, or number. It is possible to __1__ the products of some chemical ...
AQA GCSE Chemistry My Revision Notes
... (a) Suggest one reason why this part of Newlands’ table is different from the modern one. (1 mark) In 1869 Dimitri Mendeleev arranged the elements by putting them in order of their atomic weights. When he put them into a table he ensured that elements with similar properties were in columns. (b) Wha ...
... (a) Suggest one reason why this part of Newlands’ table is different from the modern one. (1 mark) In 1869 Dimitri Mendeleev arranged the elements by putting them in order of their atomic weights. When he put them into a table he ensured that elements with similar properties were in columns. (b) Wha ...
Atoms and Molecules - E
... loose from its outermost shell so that it can be stable or the combining capacity of an atom. Oxygen – Atomic number = 8; Electronic configuration = 2, 6 i.e. it has to gain 2eso that in : outer most shell has 8e-, Valency of O is -2 Similarly, valency of Al (Aluminum) is +3 (∵ electronic configurat ...
... loose from its outermost shell so that it can be stable or the combining capacity of an atom. Oxygen – Atomic number = 8; Electronic configuration = 2, 6 i.e. it has to gain 2eso that in : outer most shell has 8e-, Valency of O is -2 Similarly, valency of Al (Aluminum) is +3 (∵ electronic configurat ...
AP Chemistry - Jackson County School System
... Welcome to AP Chemistry! I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. With the ready access to hundreds of websites, I am confident that you will have sufficie ...
... Welcome to AP Chemistry! I am eagerly anticipating a great year of Chemistry. In order to ensure the best start for everyone next fall, I have prepared a summer assignment that reviews basic chemistry concepts. With the ready access to hundreds of websites, I am confident that you will have sufficie ...
CHEMONE Directions: Select the letter of the best
... a. The average kinetic energies of molecules from sample of different “ideal” gases is the same at the same temperature. b. The molecules of an ideal gas are relatively far apart. c. All molecules of an ideal gas have the same kinetic energy at constant temperature. d. Molecules of a gas undergo man ...
... a. The average kinetic energies of molecules from sample of different “ideal” gases is the same at the same temperature. b. The molecules of an ideal gas are relatively far apart. c. All molecules of an ideal gas have the same kinetic energy at constant temperature. d. Molecules of a gas undergo man ...
GCSE_C2_Revision_+_Exam_Questions
... The relative formula mass (Mr) of a compound is the sum of the relative atomic masses of the atoms in the numbers shown in the formula. The relative formula mass of a substance, in grams, is known as one mole of that ...
... The relative formula mass (Mr) of a compound is the sum of the relative atomic masses of the atoms in the numbers shown in the formula. The relative formula mass of a substance, in grams, is known as one mole of that ...
Which notation represents an atom of sodium
... 13. ____ Which statement explains why sulfur is classified as a Group 16 element? (1) A sulfur atom has 6 valence electrons. (3) Sulfur is a yellow solid at STP. (2) A sulfur atom has 16 neutrons. (4) Sulfur reacts with most metals. 14. ____ How do the atomic radius and metallic properties of sodium ...
... 13. ____ Which statement explains why sulfur is classified as a Group 16 element? (1) A sulfur atom has 6 valence electrons. (3) Sulfur is a yellow solid at STP. (2) A sulfur atom has 16 neutrons. (4) Sulfur reacts with most metals. 14. ____ How do the atomic radius and metallic properties of sodium ...
Lecture 11 - AP Chem Solutions
... 2) A potassium hydroxide solution is mixed with a solution of zinc nitrate. The potassium ion is always soluble as it is a Group 1A element. Nitrate is also soluble with everything. Thus, K+ and NO3- must be spectator ions. Zn2+(aq) + 2 OH-(aq) Æ Zn(OH)2(s) 3) A solution of lead (II) nitrate is pour ...
... 2) A potassium hydroxide solution is mixed with a solution of zinc nitrate. The potassium ion is always soluble as it is a Group 1A element. Nitrate is also soluble with everything. Thus, K+ and NO3- must be spectator ions. Zn2+(aq) + 2 OH-(aq) Æ Zn(OH)2(s) 3) A solution of lead (II) nitrate is pour ...
- Catalyst
... E) none of the above 14. What is the mass % of H in ammonium phosphate ((NH4)3PO3? A) 2.3% B) 6.0% C) 9.1% D) 17% E) none of the above 15. Naturally occurring rubidium has an atomic mass of 85.5amu. It is composed of two isotopes, rubidium–85 (84.9amu) and rubidium–87 (86.9amu). From this informatio ...
... E) none of the above 14. What is the mass % of H in ammonium phosphate ((NH4)3PO3? A) 2.3% B) 6.0% C) 9.1% D) 17% E) none of the above 15. Naturally occurring rubidium has an atomic mass of 85.5amu. It is composed of two isotopes, rubidium–85 (84.9amu) and rubidium–87 (86.9amu). From this informatio ...
Chapter 1 (Matter and Measurement) Objectives
... 2. Biological, chemical, and physical properties of matter result from the ability of atoms to form bonds from electrostatic forces between electrons and protons and between atoms and molecules. As a basis for understanding this concept: a. Students know atoms combine to form molecules by sharing el ...
... 2. Biological, chemical, and physical properties of matter result from the ability of atoms to form bonds from electrostatic forces between electrons and protons and between atoms and molecules. As a basis for understanding this concept: a. Students know atoms combine to form molecules by sharing el ...
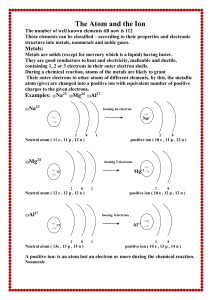
The Atom and the Ion
... Some of nonmetals are solids, others are gases and only there is one liquid element which is bromine. They have no luster, not malleable or ductile (brittle), they are bad conductors to heat and electricity, except graphite which is good conductor to electricity. Most of nonmetals contain 5,6 or 7 ...
... Some of nonmetals are solids, others are gases and only there is one liquid element which is bromine. They have no luster, not malleable or ductile (brittle), they are bad conductors to heat and electricity, except graphite which is good conductor to electricity. Most of nonmetals contain 5,6 or 7 ...
Carefully detach the last page. It is the Data Sheet.
... The vapour pressure of water is 3.17 kPa at 298 K. ...
... The vapour pressure of water is 3.17 kPa at 298 K. ...
BURNERS AND FLAMES:
... burner off, as gas will still be flowing from the main gas source and cause potential leakage. The flow of gas fuel should be started and stopped by opening or closing the source at the bench. Depending on your burner type, the supply of air entering the barrel of the burner is regulated either by a ...
... burner off, as gas will still be flowing from the main gas source and cause potential leakage. The flow of gas fuel should be started and stopped by opening or closing the source at the bench. Depending on your burner type, the supply of air entering the barrel of the burner is regulated either by a ...
Chem Review
... 20. Magnesium has 3 naturally occurring isotopes: 24Mg (23.985042amu) with a percent abundance of 78.99%, 25Mg (24.985837amu) with a percent abundance of 10.00%, and 26Mg (25.982593amu) with a percent abundance of 11.01%. Calculate the average atomic mass of Magnesium to 3 decimal places. 21. Boron ...
... 20. Magnesium has 3 naturally occurring isotopes: 24Mg (23.985042amu) with a percent abundance of 78.99%, 25Mg (24.985837amu) with a percent abundance of 10.00%, and 26Mg (25.982593amu) with a percent abundance of 11.01%. Calculate the average atomic mass of Magnesium to 3 decimal places. 21. Boron ...
Document
... A theory does not turn into a law after a long time or lots of experiments! 5. Suppose that you attempt to turn on a lamp, but the bulb does not light. Using the scientific method, describe how you might solve this problem. Be as complete as you can, and identify the elements of the scientific metho ...
... A theory does not turn into a law after a long time or lots of experiments! 5. Suppose that you attempt to turn on a lamp, but the bulb does not light. Using the scientific method, describe how you might solve this problem. Be as complete as you can, and identify the elements of the scientific metho ...
Final Review 2
... a) A maximum of one electron each b) A maximum of two electrons each c) A number of electrons that depends on the energy level. d) A number of electrons that depends on the type of orbital. 68) Which of the following elements has three valence electrons? a) lithium b) boron c) nitrogen d) more than ...
... a) A maximum of one electron each b) A maximum of two electrons each c) A number of electrons that depends on the energy level. d) A number of electrons that depends on the type of orbital. 68) Which of the following elements has three valence electrons? a) lithium b) boron c) nitrogen d) more than ...
Atomic Systems and Bonding
... atom is called a chemical element, a basic element, or just an element. Every element has a unique atomic structure. Scientists know of only about 109 basic elements at this time. (This number has a habit of changing!) All matter is composed of combinations of one or more of these elements. ...
... atom is called a chemical element, a basic element, or just an element. Every element has a unique atomic structure. Scientists know of only about 109 basic elements at this time. (This number has a habit of changing!) All matter is composed of combinations of one or more of these elements. ...
Unit 3: Bonding and Nomenclature Content Outline: Calculating
... a. You have 8.5 moles of Fluorine (F) gas. How grams of Fluorine do you have? 8.5 moles x 19.00 grams = 160.0 grams or 1.6 x102 grams 1 mole V. Calculating Percent Composition from Molar Mass A. This calculation allows for us to find the percentage (out of 100%) of one element from the total molecul ...
... a. You have 8.5 moles of Fluorine (F) gas. How grams of Fluorine do you have? 8.5 moles x 19.00 grams = 160.0 grams or 1.6 x102 grams 1 mole V. Calculating Percent Composition from Molar Mass A. This calculation allows for us to find the percentage (out of 100%) of one element from the total molecul ...
Slide 1
... oxygen for every 1.00 g of carbon carbon dioxide contains 2.67 g of oxygen for every 1.00 g of carbon since there are twice as many oxygen atoms per carbon atom in carbon dioxide than in carbon monoxide, the oxygen mass ratio should be 2 mass of oxygen that combines with 1 g of carbon in carbon diox ...
... oxygen for every 1.00 g of carbon carbon dioxide contains 2.67 g of oxygen for every 1.00 g of carbon since there are twice as many oxygen atoms per carbon atom in carbon dioxide than in carbon monoxide, the oxygen mass ratio should be 2 mass of oxygen that combines with 1 g of carbon in carbon diox ...
Chemical Equations
... reverse of a decomposition reaction. That means that two pieces join together to produce one, a more complex compound. These pieces can be elements or simpler compounds. • A + B ---> AB Reaction Types: Combustion •Combustion, at its most general, can mean the reaction of oxygen gas (O2) with anythin ...
... reverse of a decomposition reaction. That means that two pieces join together to produce one, a more complex compound. These pieces can be elements or simpler compounds. • A + B ---> AB Reaction Types: Combustion •Combustion, at its most general, can mean the reaction of oxygen gas (O2) with anythin ...
Reactions of common metals and properties of
... the amount of sodium present largely by the length of time that the intense yellow colour persists in comparison to pure sodium chloride as a standard. The potassium flame test is about five thousand times less sensitive than the sodium test. In the presence of any considerable amount of sodium it i ...
... the amount of sodium present largely by the length of time that the intense yellow colour persists in comparison to pure sodium chloride as a standard. The potassium flame test is about five thousand times less sensitive than the sodium test. In the presence of any considerable amount of sodium it i ...
Chapter 30 - The Chemical Basis of Animal Life
... Matter is anything that occupies space and has mass. It includes all of the solids, liquids, and gases in our environment, as well as those in bodies of all forms of life. Mass refers to the amount of matter in an object. Matter is composed of elements, chemical substances that ordinary chemical rea ...
... Matter is anything that occupies space and has mass. It includes all of the solids, liquids, and gases in our environment, as well as those in bodies of all forms of life. Mass refers to the amount of matter in an object. Matter is composed of elements, chemical substances that ordinary chemical rea ...
Final Velocity (V f )
... 6. Length x Width x Height will determine the ________________________ of a cube. 7. Dividing the mass by the volume will tell you the object’s ______________________. 8. 150.35km would measure the _____________________ of an object or distance between two points. 9. A __________________________ pro ...
... 6. Length x Width x Height will determine the ________________________ of a cube. 7. Dividing the mass by the volume will tell you the object’s ______________________. 8. 150.35km would measure the _____________________ of an object or distance between two points. 9. A __________________________ pro ...