Chapter 7 Chemical Formulas
... write the symbols for the ions side by side, cations first: Al3+ O22. Cross over the charges by using the absolute value of each ion’s charge as the subscript for the other ion: Al23+ O3-2 3. Check the subscripts and simplify if necessary. Final answer = Al2 O3 ...
... write the symbols for the ions side by side, cations first: Al3+ O22. Cross over the charges by using the absolute value of each ion’s charge as the subscript for the other ion: Al23+ O3-2 3. Check the subscripts and simplify if necessary. Final answer = Al2 O3 ...
Scientific Measurement
... Fusion: change from _________________ to _________________. Solidification: change from _________________ to _________________. Condensation: change from _________________ to _________________. Vaporization: change from _________________ to _________________. _____17. I can state the change of phas ...
... Fusion: change from _________________ to _________________. Solidification: change from _________________ to _________________. Condensation: change from _________________ to _________________. Vaporization: change from _________________ to _________________. _____17. I can state the change of phas ...
Unit 7 Chemical Composition: he Mole We Need to Count atoms
... Airbags are inflated by a chemical reaction: 2 NaN3(s) ...
... Airbags are inflated by a chemical reaction: 2 NaN3(s) ...
Chapter One
... • Atoms of different elements have different weights and different chemical properties. • Atoms of different elements combine in simple whole-number ratios to form compounds. • Atoms cannot be created or destroyed . When a compound is decomposed, the atoms are recovered unchanged. Dalton's assumpti ...
... • Atoms of different elements have different weights and different chemical properties. • Atoms of different elements combine in simple whole-number ratios to form compounds. • Atoms cannot be created or destroyed . When a compound is decomposed, the atoms are recovered unchanged. Dalton's assumpti ...
Final "I Can Statements" Answer Key
... Unit 2: Introduction to Matter If you can do all the things listed below, you are ready for the Unit 2 test. Place a checkmark next to each item that you can do! If a sample problem is given, complete it as evidence. _____1. I can still do everything from Unit 1. Definitions: atom – smallest partic ...
... Unit 2: Introduction to Matter If you can do all the things listed below, you are ready for the Unit 2 test. Place a checkmark next to each item that you can do! If a sample problem is given, complete it as evidence. _____1. I can still do everything from Unit 1. Definitions: atom – smallest partic ...
Chapter 2
... pure form and in combinations called compounds • Organisms are composed of matter • Matter is anything that takes up space and has mass • Matter is made up of elements ...
... pure form and in combinations called compounds • Organisms are composed of matter • Matter is anything that takes up space and has mass • Matter is made up of elements ...
Chemistry: Matter and Change
... elements form more than one compound, those compounds will have compositions that are small, whole number multiples of each other Ex. Fe2O3 and FeO – Ex. Peroxide, H2O2, and water, H2O. – Different compounds formed from the same elements. – Hydrogen mass the same in both compounds but oxygen mass is ...
... elements form more than one compound, those compounds will have compositions that are small, whole number multiples of each other Ex. Fe2O3 and FeO – Ex. Peroxide, H2O2, and water, H2O. – Different compounds formed from the same elements. – Hydrogen mass the same in both compounds but oxygen mass is ...
Possible pieces of introduction:
... that the only human experience left to him is that of humiliation and inevitable death at the hands of his captors. To Levi the value gold symbolizes is not monetary, but the deeper and fundamental urge and freedom to exercise his humanity. The story of his experience, from life in Milan to being ca ...
... that the only human experience left to him is that of humiliation and inevitable death at the hands of his captors. To Levi the value gold symbolizes is not monetary, but the deeper and fundamental urge and freedom to exercise his humanity. The story of his experience, from life in Milan to being ca ...
50 frequently forgotten facts answer key
... At equilibrium, the rates of the opposing changes are EQUAL. 39) In Le Chatelier’s Principle, if a system is at equilibrium, if something is added, then the equilibrium will shift away from the side it is on. If something is removed, then the equilibrium will shift towards that side. After the shift ...
... At equilibrium, the rates of the opposing changes are EQUAL. 39) In Le Chatelier’s Principle, if a system is at equilibrium, if something is added, then the equilibrium will shift away from the side it is on. If something is removed, then the equilibrium will shift towards that side. After the shift ...
Nitrogen`s oxidation states
... and N2O5 . The trademark of their chemistry is their ability to interconvert so it is difficult to study any one pure oxide. All of these oxides are acid anhydrides. Nitrous oxide, N2O. The proper IUPAC name N2O is dinitrogen monoxide, however its common name, nitrous oxide, is widely used. It is al ...
... and N2O5 . The trademark of their chemistry is their ability to interconvert so it is difficult to study any one pure oxide. All of these oxides are acid anhydrides. Nitrous oxide, N2O. The proper IUPAC name N2O is dinitrogen monoxide, however its common name, nitrous oxide, is widely used. It is al ...
50 Frequently Forgotten Facts Answer Key
... At equilibrium, the rates of the opposing changes are EQUAL. 39) In Le Chatelier’s Principle, if a system is at equilibrium, if something is added, then the equilibrium will shift away from the side it is on. If something is removed, then the equilibrium will shift towards that side. After the shift ...
... At equilibrium, the rates of the opposing changes are EQUAL. 39) In Le Chatelier’s Principle, if a system is at equilibrium, if something is added, then the equilibrium will shift away from the side it is on. If something is removed, then the equilibrium will shift towards that side. After the shift ...
chemistry
... plants. Boron has only two naturally occurring stable isotopes, boron-10 and boron-11. 73 Compare the abundance of the two naturally occurring isotopes of boron. [1] 74 Write an isotopic notation of the heavier isotope of the element boron. Your response must include the atomic number, the mass numb ...
... plants. Boron has only two naturally occurring stable isotopes, boron-10 and boron-11. 73 Compare the abundance of the two naturally occurring isotopes of boron. [1] 74 Write an isotopic notation of the heavier isotope of the element boron. Your response must include the atomic number, the mass numb ...
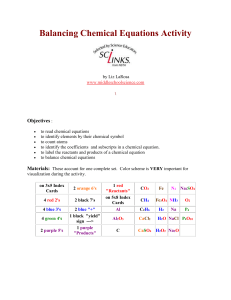
Balancing Chemical Equations Activity by Liz LaRosa www
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
physical setting chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
SCH 3U - othsmath
... ensuring the valence electrons are moved further and further from the nucleus. This increases the shielding provided by non-valence electrons, decreases the ENC (even though the number of protons in the nucleus is increasing) and causes the atomic radius to increase. Thus, the further down the group ...
... ensuring the valence electrons are moved further and further from the nucleus. This increases the shielding provided by non-valence electrons, decreases the ENC (even though the number of protons in the nucleus is increasing) and causes the atomic radius to increase. Thus, the further down the group ...
stoichiometry power point File
... • All masses are relative to 12C. This isotope of carbon was assigned a mass of 12 atomic mass units. • The most accurate method of weighing atoms is with a mass spectrometer. – Uses magnets to deflect ions. – Heavier ions are deflected less. ...
... • All masses are relative to 12C. This isotope of carbon was assigned a mass of 12 atomic mass units. • The most accurate method of weighing atoms is with a mass spectrometer. – Uses magnets to deflect ions. – Heavier ions are deflected less. ...
Introduction to Stoichiometry
... What is Stoichiometry? The proportional relationship between two or more substances during a chemical reaction. In other words, using dimensional analysis to convert one substance to another There are many different types, but they are all similar. So, let’s start small. How small? ...
... What is Stoichiometry? The proportional relationship between two or more substances during a chemical reaction. In other words, using dimensional analysis to convert one substance to another There are many different types, but they are all similar. So, let’s start small. How small? ...
File
... 7. Pure 24-karat gold has a density of 19.3 g/cm3. A bracelet is for sale at a very low price. The bracelet is 24.6 grams and has a volume of 2.8 cm3. Is this bargain bracelet pure 24karat gold? Support your answer ...
... 7. Pure 24-karat gold has a density of 19.3 g/cm3. A bracelet is for sale at a very low price. The bracelet is 24.6 grams and has a volume of 2.8 cm3. Is this bargain bracelet pure 24karat gold? Support your answer ...
H - JMap
... Wednesday, August 16, 2000 — 12:30 to 3:30 p.m., only The last page of the booklet is the answer sheet. Fold the last page along the perforations and, slowly and carefully, tear off the answer sheet. Then fill in the heading of your answer sheet. All of your answers are to be recorded on the separat ...
... Wednesday, August 16, 2000 — 12:30 to 3:30 p.m., only The last page of the booklet is the answer sheet. Fold the last page along the perforations and, slowly and carefully, tear off the answer sheet. Then fill in the heading of your answer sheet. All of your answers are to be recorded on the separat ...
chapter 1 - Louisiana Tech University
... (2) Matter is anything that has mass, occupies space, and can be seen by the naked eye. (3) The two most abundant elements in the earth’s crust are oxygen and carbon. a) All three statements are true. b) Two of the three statements are true. c) Only one of the statements is true. d) None of the stat ...
... (2) Matter is anything that has mass, occupies space, and can be seen by the naked eye. (3) The two most abundant elements in the earth’s crust are oxygen and carbon. a) All three statements are true. b) Two of the three statements are true. c) Only one of the statements is true. d) None of the stat ...
Heats of Formation WS
... 7. The Ostwald process for the commercial production of nitric acid from ammonia and oxygen involves the following steps: 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g) 2 NO (g) + O2 (g) 2 NO2 (g) 3 NO2 (g) + H2O (l) 2 HNO3 (aq) + NO (g) [a] Use the values of ∆Hfº to calculate the value of ∆Hº for ...
... 7. The Ostwald process for the commercial production of nitric acid from ammonia and oxygen involves the following steps: 4 NH3 (g) + 5 O2 (g) 4 NO (g) + 6 H2O (g) 2 NO (g) + O2 (g) 2 NO2 (g) 3 NO2 (g) + H2O (l) 2 HNO3 (aq) + NO (g) [a] Use the values of ∆Hfº to calculate the value of ∆Hº for ...
physical setting chemistry
... known, while graphite is a very soft substance. Diamond has a rigid network of bonded atoms. Graphite has atoms bonded in thin layers that are held together by weak forces. Recent experiments have produced new forms of solid carbon called fullerenes. One fullerene, C60, is a spherical, cagelike mole ...
... known, while graphite is a very soft substance. Diamond has a rigid network of bonded atoms. Graphite has atoms bonded in thin layers that are held together by weak forces. Recent experiments have produced new forms of solid carbon called fullerenes. One fullerene, C60, is a spherical, cagelike mole ...
Final Exam Review 2010 UbD
... 3. Define “matter” __Anything that has mass and volume_____________________________ 4. Define “mass” (include unit) __measures the amount of matter in an object__in grams.__________________________________________________________ 5. Define “volume” (include unit) _ measures the amount of space an ob ...
... 3. Define “matter” __Anything that has mass and volume_____________________________ 4. Define “mass” (include unit) __measures the amount of matter in an object__in grams.__________________________________________________________ 5. Define “volume” (include unit) _ measures the amount of space an ob ...
chemical reaction
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
... Chemical reactions are described by chemical equations. A chemical equation represents, with symbols and formulas, the identities and relative molecular or molar amounts of the reactants and products in a chemical reaction. For example, the following chemical equation shows that the reactant ammoni ...
AP Chemistry MC Review Questions
... (E) Wave nature of matter 18. _____Can be used to predict that a gaseous carbon atom in its ground state is paramagnetic 19. _____Explains the experimental phenomenon of electron diffraction 20. _____Indicates that an atomic orbital can hold no more than two electrons 21. _____Predicts that it is im ...
... (E) Wave nature of matter 18. _____Can be used to predict that a gaseous carbon atom in its ground state is paramagnetic 19. _____Explains the experimental phenomenon of electron diffraction 20. _____Indicates that an atomic orbital can hold no more than two electrons 21. _____Predicts that it is im ...