Chemistry I
... 100 g of carbon reacts with 133 g oxygen to carbon monoxide 100 g of carbon reacts with 266 g oxygen to carbon dioxide ...
... 100 g of carbon reacts with 133 g oxygen to carbon monoxide 100 g of carbon reacts with 266 g oxygen to carbon dioxide ...
Glossary - Chemistry (Intro)
... Dalton’s Atomic Theory: Hypotheses about the nature of matter. • Elements are composed of atoms. All atoms of a given element are identical (except for isotopes), having the same size, mass, and chemical properties. Atoms of one element are different from atoms of all other elements. • Compounds are ...
... Dalton’s Atomic Theory: Hypotheses about the nature of matter. • Elements are composed of atoms. All atoms of a given element are identical (except for isotopes), having the same size, mass, and chemical properties. Atoms of one element are different from atoms of all other elements. • Compounds are ...
chemistry
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
... Record the number of your choice for each Part A and Part B–1 multiple-choice question on your separate answer sheet. Write your answers to the Part B–2 and Part C questions in your answer booklet. All work should be written in pen, except for graphs and drawings, which should be done in pencil. You ...
Chem G 9
... neutrons will have different mass numbers and are called isotopes. Students should appreciate that a natural sample of an element is likely to contain a mixture of two or more isotopes. In determining the atomic mass of the element we must take into account that it is a mixture of isotopes with diff ...
... neutrons will have different mass numbers and are called isotopes. Students should appreciate that a natural sample of an element is likely to contain a mixture of two or more isotopes. In determining the atomic mass of the element we must take into account that it is a mixture of isotopes with diff ...
Challenge Problems
... for these elements today are very different from their accepted atomic masses at the time Döbereiner made his observations. Döbereiner also observed that strontium, calcium, and barium showed a gradual gradation in their properties, with the values of some of strontium’s properties being about midwa ...
... for these elements today are very different from their accepted atomic masses at the time Döbereiner made his observations. Döbereiner also observed that strontium, calcium, and barium showed a gradual gradation in their properties, with the values of some of strontium’s properties being about midwa ...
inorganic chemistry
... organisms in sufficient quantities. This high reactivity is due to the atoms being highly electronegative due to their high effective nuclear charge. They can gain an electron by reacting with atoms of other elements. Fluorine is one of the most reactive elements in existence, attacking otherwise in ...
... organisms in sufficient quantities. This high reactivity is due to the atoms being highly electronegative due to their high effective nuclear charge. They can gain an electron by reacting with atoms of other elements. Fluorine is one of the most reactive elements in existence, attacking otherwise in ...
Signs of Reaction - Calderglen High School
... The atoms of a compound are joined together. The substances in a mixture are NOT joined and can be easily separated. Calderglen High School ...
... The atoms of a compound are joined together. The substances in a mixture are NOT joined and can be easily separated. Calderglen High School ...
CHEMISTRY SOL REVIEW MATERIAL Name SCIENTIFIC
... 10. If you need to mix acid and water together, remember that the safety rules state that you should always add ...
... 10. If you need to mix acid and water together, remember that the safety rules state that you should always add ...
Chemical change is a process that involves recombining atoms and
... package, you break a container inside the pack that keeps the chemicals separate from each other. When they mix and react, they absorb energy and the whole mixture cools down ...
... package, you break a container inside the pack that keeps the chemicals separate from each other. When they mix and react, they absorb energy and the whole mixture cools down ...
MASS RELATIONS and STOICHIOMETRY
... Recall from Chapter 2 that atoms are never created or destroyed in a chemical reaction. Consequence: The number of atoms which were present before the reaction must be present after the reaction. A chemical equation which meets this criterion is said to be balanced. Stoichiometry is often used to ba ...
... Recall from Chapter 2 that atoms are never created or destroyed in a chemical reaction. Consequence: The number of atoms which were present before the reaction must be present after the reaction. A chemical equation which meets this criterion is said to be balanced. Stoichiometry is often used to ba ...
The Mole
... “dozen” is used to mean 12 for any object. • One mole of books is 6.022x1023 books, just like a dozen books is 12 books. ...
... “dozen” is used to mean 12 for any object. • One mole of books is 6.022x1023 books, just like a dozen books is 12 books. ...
Chemistry - Textbooks Online
... Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry studies the reactions of carbon compounds, which are 100 times more numerous than nonorganic ones. It also studies an immense variety of molecules, incl ...
... Chemistry studies the preparation, properties and reactions of all chemical elements and their compounds, except those of carbon. Organic Chemistry studies the reactions of carbon compounds, which are 100 times more numerous than nonorganic ones. It also studies an immense variety of molecules, incl ...
Chemical Equations
... Note, there appear to be more oxygen atoms, fewer hydrogen atoms at the end that at the beginning! ...
... Note, there appear to be more oxygen atoms, fewer hydrogen atoms at the end that at the beginning! ...
The Coordination Chemistry of Solvated Metal Ions in DMPU
... logical system of chemical abbreviations which essentially is the one we still use today, his contribution is simply stunning.[8-10] ...
... logical system of chemical abbreviations which essentially is the one we still use today, his contribution is simply stunning.[8-10] ...
Class XI Physical Chemistry Short note
... The main postulates of his theory: Elements are made of tiny particles called atoms. All atoms of a given element are identical. The atoms of a given element are different from those of any ...
... The main postulates of his theory: Elements are made of tiny particles called atoms. All atoms of a given element are identical. The atoms of a given element are different from those of any ...
105
... In section 10.2, you learned that a redox reaction involves changes in oxidation numbers. If an element undergoes oxidation, its oxidation number increases. If an element undergoes reduction, its oxidation number decreases. When balancing equations by the half-reaction method in section 10.3, you so ...
... In section 10.2, you learned that a redox reaction involves changes in oxidation numbers. If an element undergoes oxidation, its oxidation number increases. If an element undergoes reduction, its oxidation number decreases. When balancing equations by the half-reaction method in section 10.3, you so ...
File
... Increases across a period; increases down a group. b. Increases across a period; decreases down a group. c. Decreases across a period; increases down a group. d. Decreases across a period; decreases down a group. ...
... Increases across a period; increases down a group. b. Increases across a period; decreases down a group. c. Decreases across a period; increases down a group. d. Decreases across a period; decreases down a group. ...
Using Chemical Formulas Power ponit
... •The molar mass of a substance is equal to the mass in grams of one mole, or 6.02 x 1023 particles, of the substance. •Units for molar mass are g/mol. •The molar mass of a compound is calculated by summing the masses of the elements present in a mole of the molecules or formula units that make up th ...
... •The molar mass of a substance is equal to the mass in grams of one mole, or 6.02 x 1023 particles, of the substance. •Units for molar mass are g/mol. •The molar mass of a compound is calculated by summing the masses of the elements present in a mole of the molecules or formula units that make up th ...
CHEMISTRY – Summer Assignment Solutions 2013
... In the human body, the toxic compound hydrogen cyanide is neutralized by the acid, H 2S2O3, according to the reaction: HCN + H2S2O3 HCNS + H2SO3. If 1.000 mg of H2S2O3, is available in the body, will this be enough to neutralize 2.000 mg of HCN swallowed by a person? [hint – focus on the mole rati ...
... In the human body, the toxic compound hydrogen cyanide is neutralized by the acid, H 2S2O3, according to the reaction: HCN + H2S2O3 HCNS + H2SO3. If 1.000 mg of H2S2O3, is available in the body, will this be enough to neutralize 2.000 mg of HCN swallowed by a person? [hint – focus on the mole rati ...
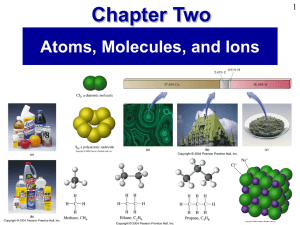
Prentice Hall Ch 02 Atoms Molecules Ions
... • Four different oxides of nitrogen can be formed by combining 28 g of nitrogen with: • 16 g oxygen, forming Compound I • 48 g oxygen, forming Compound II • 64 g oxygen, forming Compound III • 80 g oxygen, forming Compound IV What is the ratio 16:48:64:80 ...
... • Four different oxides of nitrogen can be formed by combining 28 g of nitrogen with: • 16 g oxygen, forming Compound I • 48 g oxygen, forming Compound II • 64 g oxygen, forming Compound III • 80 g oxygen, forming Compound IV What is the ratio 16:48:64:80 ...
Chem 110 2014 - University of KwaZulu
... • We use average masses in calculations, because we use large amounts of atoms and molecules in the real world. • Average atomic mass is calculated from the fractional abundance of each isotope and mass of that isotope. For example, the average atomic mass of C made up mostly of 12C (98.93%) and 13C ...
... • We use average masses in calculations, because we use large amounts of atoms and molecules in the real world. • Average atomic mass is calculated from the fractional abundance of each isotope and mass of that isotope. For example, the average atomic mass of C made up mostly of 12C (98.93%) and 13C ...
Redox
... The overall equation for a redox change can be obtained by combining the halfequations for the oxidation and reduction steps in such a way that the number of electrons donated by the reducing agent is equal to the number accepted by the oxidising agent. Any molecules or ions which appear on both sid ...
... The overall equation for a redox change can be obtained by combining the halfequations for the oxidation and reduction steps in such a way that the number of electrons donated by the reducing agent is equal to the number accepted by the oxidising agent. Any molecules or ions which appear on both sid ...