Chemistry
... introductions to all the key analytical methods including those involving advanced computerised equipment available in many analytical laboratories. The editors have built further on the work of Dr Vogel, modernising the approach while retaining the analytical concepts and ideas which were built int ...
... introductions to all the key analytical methods including those involving advanced computerised equipment available in many analytical laboratories. The editors have built further on the work of Dr Vogel, modernising the approach while retaining the analytical concepts and ideas which were built int ...
Section 1 Describing Chemical Reactions Chapter 8
... Solid aluminum carbide, Al4C3, reacts with water to produce methane gas and solid aluminum hydroxide. Write a balanced chemical equation for this reaction. ...
... Solid aluminum carbide, Al4C3, reacts with water to produce methane gas and solid aluminum hydroxide. Write a balanced chemical equation for this reaction. ...
Chemistry Review 1 Answer Key
... the boiling point of ozone is 161 K. Explain, in terms of intermolecular forces, the difference in the boiling points of O2 and O3 at standard pressure. Your response must include information about both O2 and O3. [1] O2 has a lower boiling point than O3 because the intermolecular forces between O2 ...
... the boiling point of ozone is 161 K. Explain, in terms of intermolecular forces, the difference in the boiling points of O2 and O3 at standard pressure. Your response must include information about both O2 and O3. [1] O2 has a lower boiling point than O3 because the intermolecular forces between O2 ...
Chapter 4 - profpaz.com
... This relationship is valid because the product of molarity times volume on each side equals the moles of solute, which remains constant during dilution. Molarity and volume, however, are inversely proportional during the dilution process. ...
... This relationship is valid because the product of molarity times volume on each side equals the moles of solute, which remains constant during dilution. Molarity and volume, however, are inversely proportional during the dilution process. ...
Chemistry - Resonance
... can have cyclic or ring structures. A minimum of three atoms are needed to form a ring. These compounds have been further classified into following types. (i) Alicyclic compounds : Those carbocyclic compounds which resemble to aliphatic compounds in their properties are called alicyclic compounds . ...
... can have cyclic or ring structures. A minimum of three atoms are needed to form a ring. These compounds have been further classified into following types. (i) Alicyclic compounds : Those carbocyclic compounds which resemble to aliphatic compounds in their properties are called alicyclic compounds . ...
BSC with Chemistry CBCS Syllabus 2016-17
... Practical examination will have following components: i) Performing the two practical exercises assigned by the examiner in terms of requirement of chemicals/apparatus/ theory/ reaction (if any) involved, procedure/ scheme/ observations/calculations and results. ii) viva-voce examination ii) Practic ...
... Practical examination will have following components: i) Performing the two practical exercises assigned by the examiner in terms of requirement of chemicals/apparatus/ theory/ reaction (if any) involved, procedure/ scheme/ observations/calculations and results. ii) viva-voce examination ii) Practic ...
1) A clear glass bottle contains white sand, some nails, salt water
... layer of gasoline on top. How many phases are present in this system (excluding the bottle and lid)? Four phases are mentioned: (1) white sand, (2) nails, (3) salt water with some dye dissolved in it and (4) the layer of gasoline. A 5th phase (the air) could be included since it would be above the g ...
... layer of gasoline on top. How many phases are present in this system (excluding the bottle and lid)? Four phases are mentioned: (1) white sand, (2) nails, (3) salt water with some dye dissolved in it and (4) the layer of gasoline. A 5th phase (the air) could be included since it would be above the g ...
1 Assignment 5 Hydrogen – The Unique Element
... group, it is difficult to draw comparisons between it and other elements. It exists in its elemental form as a diatomic gas, which is ...
... group, it is difficult to draw comparisons between it and other elements. It exists in its elemental form as a diatomic gas, which is ...
1 Assignment 4 Hydrogen – The Unique Element
... group, it is difficult to draw comparisons between it and other elements. It exists in its elemental form as a diatomic gas, which is ...
... group, it is difficult to draw comparisons between it and other elements. It exists in its elemental form as a diatomic gas, which is ...
Unit 5: Chemical Equations and Reactions
... Physical Change: - the change of a substance that alter its physical appearance but does not alter its chemical composition. (It is easily reversible.) Examples: freezing water into ice (phase change – freezing), breaking a large clump of sugar into powder form using a mortar and pestle. Chemical Pr ...
... Physical Change: - the change of a substance that alter its physical appearance but does not alter its chemical composition. (It is easily reversible.) Examples: freezing water into ice (phase change – freezing), breaking a large clump of sugar into powder form using a mortar and pestle. Chemical Pr ...
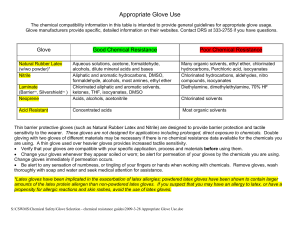
Appropriate Glove Use
... sensitivity to the wearer. These gloves are not designed for applications including prolonged, direct exposure to chemicals. Double gloving with two gloves of different materials may be necessary if there is no chemical resistance data available for the chemicals you are using. A thin glove used ove ...
... sensitivity to the wearer. These gloves are not designed for applications including prolonged, direct exposure to chemicals. Double gloving with two gloves of different materials may be necessary if there is no chemical resistance data available for the chemicals you are using. A thin glove used ove ...
Atoms and Molecules
... • Hydrogen bonds form when a hydrogen atom that is already covalently bonded to a strongly electronegative atom is attracted to another strongly electronegative atom. • These strongly electronegative atoms are typically nitrogen or oxygen. • Typically, these bonds result because the polar covalent ...
... • Hydrogen bonds form when a hydrogen atom that is already covalently bonded to a strongly electronegative atom is attracted to another strongly electronegative atom. • These strongly electronegative atoms are typically nitrogen or oxygen. • Typically, these bonds result because the polar covalent ...
Study Guide: Chemistry
... Gases - The particles are separated by greater distances and forces of attraction are virtually nonexistent which results in particles which are free to move in any direction. This causes gases to posses neither a definite volume nor shape and they occupy the whole volume of the vessel in which the ...
... Gases - The particles are separated by greater distances and forces of attraction are virtually nonexistent which results in particles which are free to move in any direction. This causes gases to posses neither a definite volume nor shape and they occupy the whole volume of the vessel in which the ...
Chemistry 11 Exam 1 Spring 2006 When answering questions be
... General Chemistry 1 Exam 2 Spring 2006 May 11, 2006 Section D01B There are 20 questions in this exam. Answer all 20, showing your reasoning where possible. Each question is valued at 5 points. Be sure to include units when reporting numerical answers. Pay attention to significant figures as well. T ...
... General Chemistry 1 Exam 2 Spring 2006 May 11, 2006 Section D01B There are 20 questions in this exam. Answer all 20, showing your reasoning where possible. Each question is valued at 5 points. Be sure to include units when reporting numerical answers. Pay attention to significant figures as well. T ...
chemistry
... prediction. If you read a description of matter which indicates that it is a solid, nonmetallic molecular compound, then (by the end of this textbook at least) you will have a good idea of its properties in general. There are many different classification systems used, some linked together, others s ...
... prediction. If you read a description of matter which indicates that it is a solid, nonmetallic molecular compound, then (by the end of this textbook at least) you will have a good idea of its properties in general. There are many different classification systems used, some linked together, others s ...
Problem 14. MAGNESIUM DETERMINATION
... extrapolation predict the following properties of the 118 th element: i) melting point; ii) boiling point, iii) atomic radius, iv) first ionization energy, v) the formula of the oxide of the 118th element in its highest oxidation state. ...
... extrapolation predict the following properties of the 118 th element: i) melting point; ii) boiling point, iii) atomic radius, iv) first ionization energy, v) the formula of the oxide of the 118th element in its highest oxidation state. ...
1. Which idea of John Dalton is no longer considered part of the
... considered part of the modern view of atoms? ...
... considered part of the modern view of atoms? ...
Chapter 19 Chemical Thermodynamics
... process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
... process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Chemical Thermodynamics © 2009, Prentice-Hall, Inc. ...
PREPARATORY PROBLEMS (Theoretical)
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
... The natural tendency of any chemical reaction to proceed in a certain direction at constant temperature and pressure is determined by the sign of the Gibbs energy of the reaction, DG. This is the universal principle. If DG < 0, the reaction can proceed predominantly in the forward direction (a produ ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.