Atomic structure and periodic table
... A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Moseley from the previous work of the Russian Scientist Dmitri Mendeleev. The horizontal arrangement forms period. Atom ...
... A periodic table is a horizontal and vertical arrangement of elements according to their atomic numbers. This table was successfully arranged in 1913 by the British scientist Henry Moseley from the previous work of the Russian Scientist Dmitri Mendeleev. The horizontal arrangement forms period. Atom ...
Spectroscopy in Organic Chemistry….
... An alternative to Energy Dispersive methods •All modern NMR and IR is done this way •Measures all frequencies at same time. More efficient at signal-gathering in a give time (better S/N) •The frequencies present are deconvoluted (or dispersed) after data is collected. •Fourier Analysis is the mathem ...
... An alternative to Energy Dispersive methods •All modern NMR and IR is done this way •Measures all frequencies at same time. More efficient at signal-gathering in a give time (better S/N) •The frequencies present are deconvoluted (or dispersed) after data is collected. •Fourier Analysis is the mathem ...
PDF of Chapter 6 Foundations of Chemistry
... Before you read, decide if you agree or disagree with each of these statements. As you read this chapter, see if you change your mind about any of the statements. 1 The atoms in all objects are the same. 2 You cannot always tell by an object’s appearance whether it is made of more than one type of a ...
... Before you read, decide if you agree or disagree with each of these statements. As you read this chapter, see if you change your mind about any of the statements. 1 The atoms in all objects are the same. 2 You cannot always tell by an object’s appearance whether it is made of more than one type of a ...
Avogadro`s Law is relation between
... a. 10–8 M c. 3.0 × 10–4 M b. 10–10 M d. 2.5 × 10–11 M 8- Calculate the value of [–OH] from the given [H3O+] and label the solution as acidic or basic. a. 10–1 M c. 2.6 × 10–7 M b. 10–13 M d. 1.2 × 10–12 M 9- Calculate the value of [H3O+] from the given [–OH] and label the solution as acidic or basic ...
... a. 10–8 M c. 3.0 × 10–4 M b. 10–10 M d. 2.5 × 10–11 M 8- Calculate the value of [–OH] from the given [H3O+] and label the solution as acidic or basic. a. 10–1 M c. 2.6 × 10–7 M b. 10–13 M d. 1.2 × 10–12 M 9- Calculate the value of [H3O+] from the given [–OH] and label the solution as acidic or basic ...
Pharmaceutical Chemistry - International Medical University
... workshops, written reports, logbooks, assignments, portfolios, presentations (oral / poster), dissertation, peer assessments, problem based learning and end-of-semester (EOS) examinations. ...
... workshops, written reports, logbooks, assignments, portfolios, presentations (oral / poster), dissertation, peer assessments, problem based learning and end-of-semester (EOS) examinations. ...
Chapter 2 Atoms, Molecules, and Ions
... • The spontaneous emission of radiation by an atom. • First observed by Henri Becquerel. • Also studied by Marie and Pierre Curie. “rays” not particles particles of some sort. Stuff comes out of atoms, “subatomic particles” Atoms, Molecules, and Ions ...
... • The spontaneous emission of radiation by an atom. • First observed by Henri Becquerel. • Also studied by Marie and Pierre Curie. “rays” not particles particles of some sort. Stuff comes out of atoms, “subatomic particles” Atoms, Molecules, and Ions ...
Redox Flash Cards - No Brain Too Small
... compounds are split into their atoms using electric currents electrolysis ...
... compounds are split into their atoms using electric currents electrolysis ...
Review AGº = -RTlnKº Calculate the equilibrium constant Kc at 25 ºC
... Because changes in enthalpy, entropy, and free energy are state functions, we can use any pathway to calculate the change in enthalpy, entropy, and free energy of an overall reaction. Hess’s Law: ΔH for a process is equal to the sum of ΔH for any set of steps, i.e., for any path that equals the over ...
... Because changes in enthalpy, entropy, and free energy are state functions, we can use any pathway to calculate the change in enthalpy, entropy, and free energy of an overall reaction. Hess’s Law: ΔH for a process is equal to the sum of ΔH for any set of steps, i.e., for any path that equals the over ...
CHOICE BASED CREDIT SYSTEM B. Sc. WITH CHEMISTRY
... Chemical Bonding and Molecular Structure Ionic Bonding: General characteristics of ionic bonding. Energy considerations in ionic bonding, lattice energy and solvation energy and their importance in the context of stability and solubility of ionic compounds. Statement of Born-Landé equation for calcu ...
... Chemical Bonding and Molecular Structure Ionic Bonding: General characteristics of ionic bonding. Energy considerations in ionic bonding, lattice energy and solvation energy and their importance in the context of stability and solubility of ionic compounds. Statement of Born-Landé equation for calcu ...
CLASS X carbon and its compound
... 1. Covalent bond or Molecular bond or Homopolar bond : A chemical bond formed between two non-metallic elements by the mutual sharing of one or more electron pairs is called covalent bond. 2. Covalency : The number of electron pairs which an atom of an element mutually shares with another atom or at ...
... 1. Covalent bond or Molecular bond or Homopolar bond : A chemical bond formed between two non-metallic elements by the mutual sharing of one or more electron pairs is called covalent bond. 2. Covalency : The number of electron pairs which an atom of an element mutually shares with another atom or at ...
9.1-10.5 Organic Chemistry
... Number the parent chain carbon atoms, starting from the end closest to the branch(es) so that the numbers are the lowest possible Identify any branches and their location number on the parent chain (us the suffix –yl for branches) If more than one of the same branch exist, use a multiplier (di, tri) ...
... Number the parent chain carbon atoms, starting from the end closest to the branch(es) so that the numbers are the lowest possible Identify any branches and their location number on the parent chain (us the suffix –yl for branches) If more than one of the same branch exist, use a multiplier (di, tri) ...
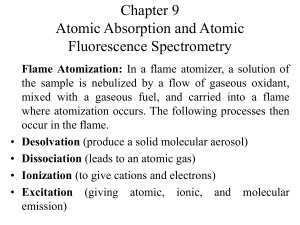
Chapter 9 Atomic Absorption and Atomic Fluorescence Spectrometry
... … Hollow Cathode Lamps continued… If the potential is sufficiently large, the ...
... … Hollow Cathode Lamps continued… If the potential is sufficiently large, the ...
CHEMISTRY – Summer Assignment Solutions 2013
... Calculate the amount of heat needed to heat 40.0 g of water, specific heat of 4.184 J/g C, from 25.0 C to 60.0 C. Treat this one of two ways. Either as a specific heat problem, use q=mTCp, where q is heat and Cp is the specific heat, T = Tfinal - Tinitial. Or, as a conversion problem, multiply ...
... Calculate the amount of heat needed to heat 40.0 g of water, specific heat of 4.184 J/g C, from 25.0 C to 60.0 C. Treat this one of two ways. Either as a specific heat problem, use q=mTCp, where q is heat and Cp is the specific heat, T = Tfinal - Tinitial. Or, as a conversion problem, multiply ...
Proposed syllabus and Scheme of Examination B.Sc. (Program) with Chemistry Submitted To
... Chemical Bonding and Molecular Structure Ionic Bonding: General characteristics of ionic bonding. Energy considerations in ionic bonding, lattice energy and solvation energy and their importance in the context of stability and solubility of ionic compounds. Statement of Born-Landé equation for calcu ...
... Chemical Bonding and Molecular Structure Ionic Bonding: General characteristics of ionic bonding. Energy considerations in ionic bonding, lattice energy and solvation energy and their importance in the context of stability and solubility of ionic compounds. Statement of Born-Landé equation for calcu ...
How do we distinguish substances?
... of important substances in our atmosphere, such as ozone and carbon dioxide, every day (see Figure 1.1). If you think about it, the fact that we can now detect or identify all of the substances present in a given system is an incredible achievement of human kind. Most of the systems we deal with, na ...
... of important substances in our atmosphere, such as ozone and carbon dioxide, every day (see Figure 1.1). If you think about it, the fact that we can now detect or identify all of the substances present in a given system is an incredible achievement of human kind. Most of the systems we deal with, na ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.