Chemistry 11 – Course Review
... c. transferred from a metal to a non-metal d. transferred from a non-metal to a metal e. closer to one end of a molecule, forming a temporary dipole ...
... c. transferred from a metal to a non-metal d. transferred from a non-metal to a metal e. closer to one end of a molecule, forming a temporary dipole ...
Chemistry Entrance Material for Grade 11 to 12
... Chemistry Entrance Material for Grade 11 to 12 ...
... Chemistry Entrance Material for Grade 11 to 12 ...
ATOMIC THEORY
... made up of invisible, _________________________ charged particles referred to as electrons. From Thomson’s experiments, scientists had to conclude that atoms were not just neutral _________________, but somehow were composed of electrically charged particles. Matter is not negatively charged, so ato ...
... made up of invisible, _________________________ charged particles referred to as electrons. From Thomson’s experiments, scientists had to conclude that atoms were not just neutral _________________, but somehow were composed of electrically charged particles. Matter is not negatively charged, so ato ...
contents 2002 MAY
... critical micelle concentration (CMC) is explained in terms of their condensation /penetration onto/into the micelles. The decrease in CMC and increase in counter-ion binding of cationic surfactants is attributed to the increased hydrophobicity in alkyl chain of counter-ions. In the case of monocarbo ...
... critical micelle concentration (CMC) is explained in terms of their condensation /penetration onto/into the micelles. The decrease in CMC and increase in counter-ion binding of cationic surfactants is attributed to the increased hydrophobicity in alkyl chain of counter-ions. In the case of monocarbo ...
IX Chemistry Chapter 02
... combined. The French Chemist Lavosier, (1785) tried to learn about chemical changes by weighing the quantities of substances used in chemical reactions. He found that when a chemical reaction was carried out in a closed system, the total weight of the system was not changed. The most important chemi ...
... combined. The French Chemist Lavosier, (1785) tried to learn about chemical changes by weighing the quantities of substances used in chemical reactions. He found that when a chemical reaction was carried out in a closed system, the total weight of the system was not changed. The most important chemi ...
Many Chemistries Could Be Used to Build Living Systems
... The other basic feature of life is its ability to capture and transform energy to power this process. The accuracy of this replication is primarily a function of how much energy is expended in getting it right, showing the centrality of energetics to living. Life requires many other functions, such ...
... The other basic feature of life is its ability to capture and transform energy to power this process. The accuracy of this replication is primarily a function of how much energy is expended in getting it right, showing the centrality of energetics to living. Life requires many other functions, such ...
fahad h. ahmad - Fahad`s Academy
... Where x = distance moved by the substance and; y = distance moved by the solvent Checking the Purity of Substances - Pure substances have FIXED MELTING AND BOILING POINTS. Pure water boils at 100oC and melts at 0oC. - Impure substances have NO FIXED MELTING AND BOILING POINTS. They melt and boil a ...
... Where x = distance moved by the substance and; y = distance moved by the solvent Checking the Purity of Substances - Pure substances have FIXED MELTING AND BOILING POINTS. Pure water boils at 100oC and melts at 0oC. - Impure substances have NO FIXED MELTING AND BOILING POINTS. They melt and boil a ...
Revision of the ACS Guidelines for Undergraduate Chemistry
... specialized degree tracks that focus on a specific chemistry subdiscipline or provide an interdisciplinary experience, thereby replacing the previous ACS-approved degree options. The total number of laboratory hours required will be 400 beyond the introductory chemistry experience, with at least 180 ...
... specialized degree tracks that focus on a specific chemistry subdiscipline or provide an interdisciplinary experience, thereby replacing the previous ACS-approved degree options. The total number of laboratory hours required will be 400 beyond the introductory chemistry experience, with at least 180 ...
The Mole: A Measurement of Matter
... You live in a quantitative world. The grade you got on your last exam, the number of times you heard your favorite song on the radio yesterday, and the cost of a bicycle you would like to own are all important quantities to you. These are quantities that answer questions such as "How much?" or "How ...
... You live in a quantitative world. The grade you got on your last exam, the number of times you heard your favorite song on the radio yesterday, and the cost of a bicycle you would like to own are all important quantities to you. These are quantities that answer questions such as "How much?" or "How ...
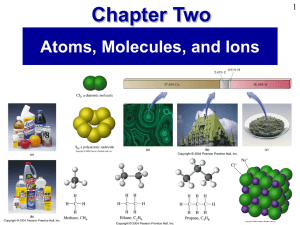
Prentice Hall Ch 02 Atoms Molecules Ions
... We usually write mass ratios in a form such as “the ratio of O to Mg is 0.6583:1.” The first number represents the mass of the first element named—in this case, a mass of oxygen, say 0.6583 g oxygen—and the second number represents the mass of the second element named—here a mass of magnesium. Altho ...
... We usually write mass ratios in a form such as “the ratio of O to Mg is 0.6583:1.” The first number represents the mass of the first element named—in this case, a mass of oxygen, say 0.6583 g oxygen—and the second number represents the mass of the second element named—here a mass of magnesium. Altho ...
Group 1: The Alkali Metals
... used mostly to produce chemicals, such as fertilizers for use in agriculture. o Potassium is an important nutrient needed for plant growth. ...
... used mostly to produce chemicals, such as fertilizers for use in agriculture. o Potassium is an important nutrient needed for plant growth. ...
2015_Final Exam Study Guide
... b. absorbed if the solution is solid. c. exothermic if solvation forces are higher than the forces required to separate solute particles and solvent molecules. d. Answers a and c are both correct. Which of the following is not an important factor influencing solubility? a. chemical nature of solute ...
... b. absorbed if the solution is solid. c. exothermic if solvation forces are higher than the forces required to separate solute particles and solvent molecules. d. Answers a and c are both correct. Which of the following is not an important factor influencing solubility? a. chemical nature of solute ...
Document
... Hydrogen ions, H+(aq), make solutions acidic and hydroxide ions, OH–(aq), make solutions alkaline. The pH scale is a measure of the acidity or alkalinity of a solution. In neutralisation reactions, hydrogen ions react with hydroxide ions to produce water. This reaction can be represented by the equa ...
... Hydrogen ions, H+(aq), make solutions acidic and hydroxide ions, OH–(aq), make solutions alkaline. The pH scale is a measure of the acidity or alkalinity of a solution. In neutralisation reactions, hydrogen ions react with hydroxide ions to produce water. This reaction can be represented by the equa ...
Unit 3 - High School Chemistry
... structure of an ionic compound, they are not referred to as molecules. 3. Ionic solids are generally High Melting Points (typically 300°C to 1000°C). Since a strong force can only shatter the crystal but not bend it as in metals, the energy needed to completely break up the lattice structure (lattic ...
... structure of an ionic compound, they are not referred to as molecules. 3. Ionic solids are generally High Melting Points (typically 300°C to 1000°C). Since a strong force can only shatter the crystal but not bend it as in metals, the energy needed to completely break up the lattice structure (lattic ...
Indian Journal of Chemistry
... in the activation barrier (δmΔG≠) is measured. Solvent effects on reactivity trends for base hydrolysis of these compounds have been analysed into initial state and transition state components which are determined from transfer chemical potentials and kinetic data. It is observed that the substituen ...
... in the activation barrier (δmΔG≠) is measured. Solvent effects on reactivity trends for base hydrolysis of these compounds have been analysed into initial state and transition state components which are determined from transfer chemical potentials and kinetic data. It is observed that the substituen ...
CHEMICAL REACTIONS
... • Take one element at a time usually starting with the most complex substance. • It is usually better to balance in this order: metals, nonmetals, hydrogen, oxygen. • If everything balances except for O2, and there is no way to balance O2 with a whole number, use a fraction or mixed number. Then, mu ...
... • Take one element at a time usually starting with the most complex substance. • It is usually better to balance in this order: metals, nonmetals, hydrogen, oxygen. • If everything balances except for O2, and there is no way to balance O2 with a whole number, use a fraction or mixed number. Then, mu ...
Answers to Selected Problems
... 67. Answers will vary. The model of the atom uses the abstract idea of probability; light is considered a particle and a wave at the same time. Atoms and light cannot be compared to familiar objects or observations because humans cannot experience atoms or photons directly and because matter and ene ...
... 67. Answers will vary. The model of the atom uses the abstract idea of probability; light is considered a particle and a wave at the same time. Atoms and light cannot be compared to familiar objects or observations because humans cannot experience atoms or photons directly and because matter and ene ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.