Chapter 1 Chemistry: The Study of Matter
... Gas in gas- air Solid in solid - brass Liquid in gas- water vapor ...
... Gas in gas- air Solid in solid - brass Liquid in gas- water vapor ...
rev8thgrade - PAMS
... A force is a push or pull. Force is measured in newtons. Force can cause objects to move, stop moving, change speed, or change direction. Speed is the change in position of an object per unit of time. Velocity may have a positive or a negative value depending on the direction of the change in positi ...
... A force is a push or pull. Force is measured in newtons. Force can cause objects to move, stop moving, change speed, or change direction. Speed is the change in position of an object per unit of time. Velocity may have a positive or a negative value depending on the direction of the change in positi ...
Chapter 1 Chemistry: The Study of Matter
... Heat- the energy that moves because of a temperature difference. Chemical energy- energy released or absorbed in a chemical change. Electrical energy - energy of moving ...
... Heat- the energy that moves because of a temperature difference. Chemical energy- energy released or absorbed in a chemical change. Electrical energy - energy of moving ...
Slide 1
... The mass ratio for one of the elements that combines with a fixed mass of another element can be expressed in small whole numbers ...
... The mass ratio for one of the elements that combines with a fixed mass of another element can be expressed in small whole numbers ...
Chapter 4 Study Guide-Atomic Structure Define the following terms
... Periodic Table-arrangement of elements in which the elements are separated into groups based on a set of repeating properties Proton-positively charged subatomic particle that lives in the nucleus Plum Pudding Model-electrons stuck in positive lump (JJ Thomson) ...
... Periodic Table-arrangement of elements in which the elements are separated into groups based on a set of repeating properties Proton-positively charged subatomic particle that lives in the nucleus Plum Pudding Model-electrons stuck in positive lump (JJ Thomson) ...
Chemical Reactions
... • A + sign separates molecules on the same side • The arrow is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen react to yield carbon dioxide” ...
... • A + sign separates molecules on the same side • The arrow is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen react to yield carbon dioxide” ...
All That Matters - Teach-n-Learn-Chem
... One of the many ways scientist classify substances is by the periodic table. The periodic table is a chart showing the elements in order by increasing atomic number and grouped by their similar qualities. Russian chemist Dimitri Mendeleev is believed to be the first to place the elements in order by ...
... One of the many ways scientist classify substances is by the periodic table. The periodic table is a chart showing the elements in order by increasing atomic number and grouped by their similar qualities. Russian chemist Dimitri Mendeleev is believed to be the first to place the elements in order by ...
CLASS NOTES- Balancing Chemical Equations.pptx
... changes of substances • The parts of a chemical reaction Learners will be able to… • Write and balance chemical equations • Perform stoichiometry calculations ...
... changes of substances • The parts of a chemical reaction Learners will be able to… • Write and balance chemical equations • Perform stoichiometry calculations ...
chapter 2
... 6. What is a physical change? a change during which some properties of a material change, but the composition of the material does not change Example: ___ tear/rip _________ ...
... 6. What is a physical change? a change during which some properties of a material change, but the composition of the material does not change Example: ___ tear/rip _________ ...
Chapters 1-4 Numbers and Measurements in Chemistry Units SI
... What is the mass of a cube of osmium that is 32 mm on each side? ...
... What is the mass of a cube of osmium that is 32 mm on each side? ...
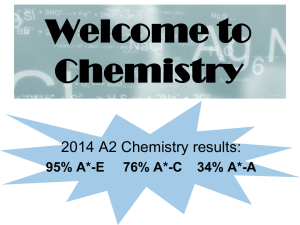
A Level Chemistry.pub
... • This means if you want a full A Level you will need to decide that at the start of your course. • You will still be able to combine A Levels with other types of qualifications such as BTECs. • These changes are happening at different times for different subjects. • You’ll have lots of support from ...
... • This means if you want a full A Level you will need to decide that at the start of your course. • You will still be able to combine A Levels with other types of qualifications such as BTECs. • These changes are happening at different times for different subjects. • You’ll have lots of support from ...
AP Chemistry Placement Test To be successful in AP Chemistry
... test. The test covers some important math skills and topics covered in the first half of introductory chemistry such as definitions of elements, compounds, mixtures, atoms, molecules, ions and types of bonds. Problems involving percentages, density, atomic structure, formulas, molecular weight, mole ...
... test. The test covers some important math skills and topics covered in the first half of introductory chemistry such as definitions of elements, compounds, mixtures, atoms, molecules, ions and types of bonds. Problems involving percentages, density, atomic structure, formulas, molecular weight, mole ...
Name - Quia
... Chemistry Midterm review –random extra questions *Test may cover other topics not included on this review, yet have been covered throughout the semester. ...
... Chemistry Midterm review –random extra questions *Test may cover other topics not included on this review, yet have been covered throughout the semester. ...
april test
... excess dilute H2SO4 and 1.910 L of H2 gas was evolved and collected over water at 23°C and 746 mm of Hg. Calculate the atomic weight of Al. (Vapour pressure of H2O at 23°C = 21.0 mmHg) Al(s) + H2SO4(aq) Al2(SO4)3(aq) + H2(g) (unbalanced) ...
... excess dilute H2SO4 and 1.910 L of H2 gas was evolved and collected over water at 23°C and 746 mm of Hg. Calculate the atomic weight of Al. (Vapour pressure of H2O at 23°C = 21.0 mmHg) Al(s) + H2SO4(aq) Al2(SO4)3(aq) + H2(g) (unbalanced) ...
Document
... This claim is false because it — F violates the principle of constant composition G contradicts the law of conservation of matter H ignores the strength of the theory of strings J violates the rules of gravitational attraction ...
... This claim is false because it — F violates the principle of constant composition G contradicts the law of conservation of matter H ignores the strength of the theory of strings J violates the rules of gravitational attraction ...
Camp 1 - Quynh Nguyen Official Website
... Pure substance that cannot be decomposed into other pure substances by ordinary chemical means. Atom Smallest particle of an element that can combine with atoms of other elements to form compounds. Compound Pure substance that can be broken down into two or more other pure substances by a chemical c ...
... Pure substance that cannot be decomposed into other pure substances by ordinary chemical means. Atom Smallest particle of an element that can combine with atoms of other elements to form compounds. Compound Pure substance that can be broken down into two or more other pure substances by a chemical c ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.