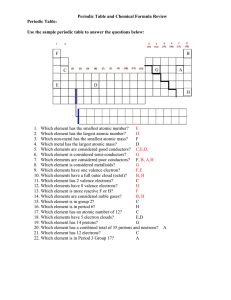
Periodic Table Review Key
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
High School Chemistry
... Science Benchmark In a chemical reaction new substances are formed as atoms and molecules are rearranged. The concept of atoms accounts for the conservation of mass since the number of atoms stays the same in a chemical reaction. Energy can be absorbed or released in a chemical reaction, but the tot ...
... Science Benchmark In a chemical reaction new substances are formed as atoms and molecules are rearranged. The concept of atoms accounts for the conservation of mass since the number of atoms stays the same in a chemical reaction. Energy can be absorbed or released in a chemical reaction, but the tot ...
CHAPTER 2: ATOMS, IONS, AND COMPOUNDS
... CHAPTER 2: ATOMS, IONS, AND COMPOUNDS (Topics to Review) Early Models Democritus (462-370 B.C.): proposed that all matter was made up of tiny, indivisible particles called atomos (meaning “not to cut) or atoms. Empedocles (490-430 B.C.): suggested all matter was composed of four basic elements: air, ...
... CHAPTER 2: ATOMS, IONS, AND COMPOUNDS (Topics to Review) Early Models Democritus (462-370 B.C.): proposed that all matter was made up of tiny, indivisible particles called atomos (meaning “not to cut) or atoms. Empedocles (490-430 B.C.): suggested all matter was composed of four basic elements: air, ...
Chapter 2 - Chemistry
... - basic theory of modern chemistry - all matter whether element, compound or mixture is composed of small particles called atoms - purpose of atomic theory: to provide explanation of the structure of matter in terms of different combinations of very small particles ...
... - basic theory of modern chemistry - all matter whether element, compound or mixture is composed of small particles called atoms - purpose of atomic theory: to provide explanation of the structure of matter in terms of different combinations of very small particles ...
Chapter-1-Intro - Mister Chemistry Welcomes You!
... (slides that follow are linked to earlier ones) ...
... (slides that follow are linked to earlier ones) ...
Intro to Chapter 5 Development of the Periodic Table
... example of how scientific theory comes into being. Through random observations followed by organization of data into trends resulted in a consistent hypothesis which could explain known facts and makes correct predictions of the elements. B. Mendeleev s organized chemical information by: 1) listing ...
... example of how scientific theory comes into being. Through random observations followed by organization of data into trends resulted in a consistent hypothesis which could explain known facts and makes correct predictions of the elements. B. Mendeleev s organized chemical information by: 1) listing ...
Midterm Review - Closter Public Schools
... A ______________ is a pure substance that contains only a single type of atom. A ______________ is a pure substance that consists of two or more different types of atoms bonded together. A ______________ is a combination of different substances that remain the same individual substances and can be s ...
... A ______________ is a pure substance that contains only a single type of atom. A ______________ is a pure substance that consists of two or more different types of atoms bonded together. A ______________ is a combination of different substances that remain the same individual substances and can be s ...
Matter - Moodle
... • Generally ___________ as easy to observe as physical properties • The chemical composition ______________________ A chemical property describes how a substance ________________ into a new substance Either by: • __________________ with other elements • _________________ __________________ into new ...
... • Generally ___________ as easy to observe as physical properties • The chemical composition ______________________ A chemical property describes how a substance ________________ into a new substance Either by: • __________________ with other elements • _________________ __________________ into new ...
Atoms, Molecules and Ions
... 2,000,000 : Home species diverged from A. 250,000 : Homo Sapience – Stone Tool. 80,000 : Fire (Cooking) 10,000 : Farming, Neolithic Age 7,000 : Copper 6,000 : Wine 5,000 : Bronze 3,300 : Iron 2,400: Atomic Theory(Democritus) 2,000: (Alchemy) 500: Metallurgy (Bauer), Alchemy (Paracelsus ) 400: Modern ...
... 2,000,000 : Home species diverged from A. 250,000 : Homo Sapience – Stone Tool. 80,000 : Fire (Cooking) 10,000 : Farming, Neolithic Age 7,000 : Copper 6,000 : Wine 5,000 : Bronze 3,300 : Iron 2,400: Atomic Theory(Democritus) 2,000: (Alchemy) 500: Metallurgy (Bauer), Alchemy (Paracelsus ) 400: Modern ...
30.09.2013 1 Chapter 2 Atoms and Molecules Warning!! Chapter
... • Compounds have different properties than their constituent atoms. • Ionic compounds contain cations and anions, usually arranged in a lattice. • Molecular formulas indicate the elements and number of atoms of each element actually contained in a discrete unit of a compound. • Empirical formulas in ...
... • Compounds have different properties than their constituent atoms. • Ionic compounds contain cations and anions, usually arranged in a lattice. • Molecular formulas indicate the elements and number of atoms of each element actually contained in a discrete unit of a compound. • Empirical formulas in ...
Chemistry Note PowerPoint
... What is a chemical bond? • A chemical bond is the force of attraction between two atoms, when they combine. • Atoms combine to form larger particles called molecules • A molecule is simply two or more atoms held together by a chemical bond • Oxygen molecule ...
... What is a chemical bond? • A chemical bond is the force of attraction between two atoms, when they combine. • Atoms combine to form larger particles called molecules • A molecule is simply two or more atoms held together by a chemical bond • Oxygen molecule ...
Matter_and_Change2
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
... Matter with a uniform and definite composition (also called a pure substance). All samples of a substance have identical physical properties. ...
Lecture 2 - Columbia University
... indestructible, namely those atoms which according to our teaching are the seeds or prime units of things from which the whole universe is ...
... indestructible, namely those atoms which according to our teaching are the seeds or prime units of things from which the whole universe is ...
Year 11 Chemistry Balancing Equations
... How do the number of protons, number of neutrons, and the mass number relate to each other? ...
... How do the number of protons, number of neutrons, and the mass number relate to each other? ...
matter - Firelands Local Schools
... b. A compound is different from the elements that comprise it, while a mixture may have some of the properties similar to the pure substances that make it c. Heterogeneous mixture: a mixture that substances aren’t uniformly mixed 1. Example: flour and water d. Homogeneous mixture: a mixture that su ...
... b. A compound is different from the elements that comprise it, while a mixture may have some of the properties similar to the pure substances that make it c. Heterogeneous mixture: a mixture that substances aren’t uniformly mixed 1. Example: flour and water d. Homogeneous mixture: a mixture that su ...
Chem 1411 Chapt2
... 4. Compounds are formed when atoms of more than one type of element combine. Law of Multiple Proportions- If two substances are made of the same types of elements, but the elements are in different proportions, then the two substances are different. Example: NO, NO2, N2O, H2O, H2O2 Law of Constant C ...
... 4. Compounds are formed when atoms of more than one type of element combine. Law of Multiple Proportions- If two substances are made of the same types of elements, but the elements are in different proportions, then the two substances are different. Example: NO, NO2, N2O, H2O, H2O2 Law of Constant C ...
Atomic number
... Atomic number: the number of protons in an atom. All elements are identified by their atomic number. For example, any element with 6 protons is Carbon, regardless of how many neutrons (or electrons) it has Atomic mass: the sum of the number of protons and neutrons. ...
... Atomic number: the number of protons in an atom. All elements are identified by their atomic number. For example, any element with 6 protons is Carbon, regardless of how many neutrons (or electrons) it has Atomic mass: the sum of the number of protons and neutrons. ...
Carbon-12 Stable
... Often the appearance (texture, color, physical state) can change. Other signs of change include… ...
... Often the appearance (texture, color, physical state) can change. Other signs of change include… ...
Chemistry Comes Alive: Part A
... • Molarity, or moles per liter (M) • 1 mole = the atomic weight of an element or molecular weight (sum of atomic weights) of a compound in grams • 1 mole of any substance contains 6.02 1023 molecules (Avogadro’s number) ...
... • Molarity, or moles per liter (M) • 1 mole = the atomic weight of an element or molecular weight (sum of atomic weights) of a compound in grams • 1 mole of any substance contains 6.02 1023 molecules (Avogadro’s number) ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.