Chapter 2 - Molecules of Life (Biochemistry) Periodic Table of
... • Relatively weak bonds, but lots of them together can be strong. ! • Result from unequal sharing of electrons in polar covalent molecules.! • Partial positive and negative charges on different molecules attract each other.! Water is a polar covalent molecule. ! • Electrons are shared unequally ...
... • Relatively weak bonds, but lots of them together can be strong. ! • Result from unequal sharing of electrons in polar covalent molecules.! • Partial positive and negative charges on different molecules attract each other.! Water is a polar covalent molecule. ! • Electrons are shared unequally ...
Chapter 4
... Radioactivity ■ In the late 1890’s Scientists noticed some substances spontaneously emitted radiation in a process called radioactivity. This is because their nuclei is unstable ■ Rays and particles emitted are called radiation ■ Radioactive atoms undergo changes that alters their identity and allo ...
... Radioactivity ■ In the late 1890’s Scientists noticed some substances spontaneously emitted radiation in a process called radioactivity. This is because their nuclei is unstable ■ Rays and particles emitted are called radiation ■ Radioactive atoms undergo changes that alters their identity and allo ...
Radioactive Reactions
... • Fission: atomic nucleus splits into parts (smaller). The particles leave the nucleus causing energy to be released. The atomic bomb and nuclear reactors work by fission • Fusion: atomic nucleus gets larger because particles are coming into the nucleus. In the Sun, hydrogen nuclei fuse to make heli ...
... • Fission: atomic nucleus splits into parts (smaller). The particles leave the nucleus causing energy to be released. The atomic bomb and nuclear reactors work by fission • Fusion: atomic nucleus gets larger because particles are coming into the nucleus. In the Sun, hydrogen nuclei fuse to make heli ...
Chemistry Fall Final Review 2012-2013 Alchemy Unit
... 11. What are ions? What are cations and anions? Ions are atoms that have lost or gain electrons and become positive or negative charged. Cation is a positive ion and an anion is a negative ion. 12. In ionic bonds, metals tend to lose electrons and nonmetals gain electrons. What happens to these elem ...
... 11. What are ions? What are cations and anions? Ions are atoms that have lost or gain electrons and become positive or negative charged. Cation is a positive ion and an anion is a negative ion. 12. In ionic bonds, metals tend to lose electrons and nonmetals gain electrons. What happens to these elem ...
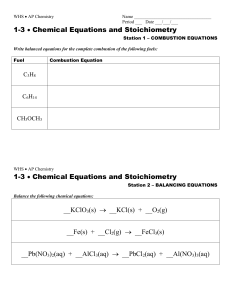
Chemical Equations
... Reactants are on the left. Products are on the right. The arrow means “produces” or “changes into”. The letters in parentheses are the physical states of the substances: (s) -- solid (l) -- liquid (g) -- gas (aq) -- aqueous ...
... Reactants are on the left. Products are on the right. The arrow means “produces” or “changes into”. The letters in parentheses are the physical states of the substances: (s) -- solid (l) -- liquid (g) -- gas (aq) -- aqueous ...
The Periodic table
... A region of space within an electron subshell where an electron with a specific energy is most likely to be found. S subshell=1 orbital, p subshell=3 orbitals, d subshell=5 orbitals, f subshell=7 orbitals. Maximum number of electrons in a subshell is always 2. S orbital=spherical, p orbital ...
... A region of space within an electron subshell where an electron with a specific energy is most likely to be found. S subshell=1 orbital, p subshell=3 orbitals, d subshell=5 orbitals, f subshell=7 orbitals. Maximum number of electrons in a subshell is always 2. S orbital=spherical, p orbital ...
Chapter 2
... – There had to be a source of positive charge because the atom is neutral. – Thomson assumed there were no positively charged pieces because none showed up in the cathode ray experiment. ...
... – There had to be a source of positive charge because the atom is neutral. – Thomson assumed there were no positively charged pieces because none showed up in the cathode ray experiment. ...
Chemistry 30 Notes - Heat of Formation February 2nd
... The heat of formation for pure elements, such as H2(g), O2(g), Al(s), etc. is 0 kJ·mole-1. You'll find it useful to remember this. ...
... The heat of formation for pure elements, such as H2(g), O2(g), Al(s), etc. is 0 kJ·mole-1. You'll find it useful to remember this. ...
Atomic Structure - Hudson City School District
... • Water would not condense from vapor into solid or liquid forms if its molecules didn't attract each other. • Many properties of molecular compounds, including crystal structures (e. g. the shapes of snowflakes), melting points, boiling points, heats of fusion and vaporization, surface tension, and ...
... • Water would not condense from vapor into solid or liquid forms if its molecules didn't attract each other. • Many properties of molecular compounds, including crystal structures (e. g. the shapes of snowflakes), melting points, boiling points, heats of fusion and vaporization, surface tension, and ...
The Periodic Table - Mrs Molchany`s Webpage
... Most non-metallic oxides are molecular substances that form acidic solutions Tend to form anions or oxyanions in aqueous solution ...
... Most non-metallic oxides are molecular substances that form acidic solutions Tend to form anions or oxyanions in aqueous solution ...
File
... experimental observations made by several scientists. Three concepts of Dalton’s atomic theory are stated below. Statement A: Atoms are indivisible and cannot be destroyed or broken down into smaller parts. Statement B: Atoms of one element cannot be changed into atoms of another element. Statement ...
... experimental observations made by several scientists. Three concepts of Dalton’s atomic theory are stated below. Statement A: Atoms are indivisible and cannot be destroyed or broken down into smaller parts. Statement B: Atoms of one element cannot be changed into atoms of another element. Statement ...
CHEM 121 Chp 2 Spaulding
... Elements in the same group have the same number of valence electrons. The group number, 1A–8A, equals the number of valence electrons for the main group elements. The exception is He, which has only 2 valence ...
... Elements in the same group have the same number of valence electrons. The group number, 1A–8A, equals the number of valence electrons for the main group elements. The exception is He, which has only 2 valence ...
Fundamentals of Chemistry
... • The number of electrons in the valence shell determines the relative activity of an element. • The arrangement of electrons in the outer shell explains why some elements are chemically very active, some are not very active, and others are inert. • Group I has 1 valence electron, which makes it eas ...
... • The number of electrons in the valence shell determines the relative activity of an element. • The arrangement of electrons in the outer shell explains why some elements are chemically very active, some are not very active, and others are inert. • Group I has 1 valence electron, which makes it eas ...
chemistry i
... 7. What is the chemical formula for a compound formed from calcium ions (Ca2-) and chloride ions(Cl–)? a. CaCl b. Ca2Cl c. CaCl2 d. Ca2Cl2 8. What is the mass number of an atom which contains 28 protons, 28 electrons, and 34 neutrons? A. 28 B. 56 C. 62 D. 90 9. According to the Chemistry Reference T ...
... 7. What is the chemical formula for a compound formed from calcium ions (Ca2-) and chloride ions(Cl–)? a. CaCl b. Ca2Cl c. CaCl2 d. Ca2Cl2 8. What is the mass number of an atom which contains 28 protons, 28 electrons, and 34 neutrons? A. 28 B. 56 C. 62 D. 90 9. According to the Chemistry Reference T ...
Utah - Wavefunction, Inc.
... The nucleus of an atom is a tiny fraction of the volume of the atom. Each proton or neutron in the nucleus is nearly 2,000 times the mass of an electron. Electrons move around the nucleus. The modern atomic model has been developed using experimental evidence. Atomic theories describe th ...
... The nucleus of an atom is a tiny fraction of the volume of the atom. Each proton or neutron in the nucleus is nearly 2,000 times the mass of an electron. Electrons move around the nucleus. The modern atomic model has been developed using experimental evidence. Atomic theories describe th ...
4 - College of Arts and Sciences
... Reduce electrons by putting in a double bond |C = N| Count electrons. 12 Correct number? No! Now what? ...
... Reduce electrons by putting in a double bond |C = N| Count electrons. 12 Correct number? No! Now what? ...
Honors Biology Chapter 2 Power Point
... spontaneously to emit (give off) particles or energy until it is stable • Here a radioisotope is used to examine a thyroid gland • Radioactive Tracers in Medicine (3:57) ...
... spontaneously to emit (give off) particles or energy until it is stable • Here a radioisotope is used to examine a thyroid gland • Radioactive Tracers in Medicine (3:57) ...
practice final examination
... 10. Answer true or false for each of the following questions below (circle your choice): a) ...
... 10. Answer true or false for each of the following questions below (circle your choice): a) ...
Chemistry: Matter and Change
... • A compound is a made up of two or more elements combined chemically. • Most of the matter in the universe exists as compounds. ...
... • A compound is a made up of two or more elements combined chemically. • Most of the matter in the universe exists as compounds. ...
CHAPTER 1: INTRODUCTION TO ENVIRONMENTAL CHEMISTRY
... Environmental chemists participate in all aspects of this process from collecting the data for basic research, to monitoring environmental quality to developing chemical processes for remediation and environmental cleanup. Environmental chemists work at the interface of chemistry with biologists, ge ...
... Environmental chemists participate in all aspects of this process from collecting the data for basic research, to monitoring environmental quality to developing chemical processes for remediation and environmental cleanup. Environmental chemists work at the interface of chemistry with biologists, ge ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.