Unit 2 - Biochemistry Notes
... Compound – when different elements combine. CO2 and H2O are molecules, but they are also compounds because they are molecules containing more than one element. ...
... Compound – when different elements combine. CO2 and H2O are molecules, but they are also compounds because they are molecules containing more than one element. ...
Organic Chemistry
... Organic Chemistry: What is it? • 1780: Organic compounds are very complex and only obtained from living sources (vitalism 生机说) • Vitalism: Belief that a "magic" vital force, present in plants and animals, is necessary for the synthesis of organic compounds • 1789: Antoine Laurent Lavoisier observed ...
... Organic Chemistry: What is it? • 1780: Organic compounds are very complex and only obtained from living sources (vitalism 生机说) • Vitalism: Belief that a "magic" vital force, present in plants and animals, is necessary for the synthesis of organic compounds • 1789: Antoine Laurent Lavoisier observed ...
CHEM_S1CourseReview_2011
... Why are significant figures important to chemists? What is the best method/graph to represent specific data? How would a scientist organize data collected from an experiment into a graph? How does a standard notation number compare to a scientific notation number? How do you differentiate ...
... Why are significant figures important to chemists? What is the best method/graph to represent specific data? How would a scientist organize data collected from an experiment into a graph? How does a standard notation number compare to a scientific notation number? How do you differentiate ...
In organic chemistry, we studied a lot about the essential elements
... writing about “CHEMISTRY IN LIFE”. I was confused what I was going to write about chemistry? It is basically unfamiliar to me at the first place. But that was my assignment which I have to get it done. Therefore I started looking, checking, asking, etc. about chemistry. I was looking things at thin ...
... writing about “CHEMISTRY IN LIFE”. I was confused what I was going to write about chemistry? It is basically unfamiliar to me at the first place. But that was my assignment which I have to get it done. Therefore I started looking, checking, asking, etc. about chemistry. I was looking things at thin ...
The Periodic Table
... Most non-metallic oxides are molecular substances that form acidic solutions ...
... Most non-metallic oxides are molecular substances that form acidic solutions ...
Unit C3, C3.1
... However, other chemists did not accept Newlands’ ideas. It was not until much later that his contribution to the development of the modern periodic table was recognised. Reproduced courtesy of the library and information centre Royal Society of Chemistry ...
... However, other chemists did not accept Newlands’ ideas. It was not until much later that his contribution to the development of the modern periodic table was recognised. Reproduced courtesy of the library and information centre Royal Society of Chemistry ...
Chemistry 515 Name: L. S. Curtin Soc. Sec. #: February 8, 1999
... The correctly balanced equation is: 2Al(s) + 3 Cl2(g) → 2 AlCl3(s) 11) Which of the following statements about Daltons Atomic Theory has been shown to be incorrect? a) b) c) d) e) ...
... The correctly balanced equation is: 2Al(s) + 3 Cl2(g) → 2 AlCl3(s) 11) Which of the following statements about Daltons Atomic Theory has been shown to be incorrect? a) b) c) d) e) ...
Single-Replacement Reactions
... Balance the atoms of an element one at a time by adding coefficients (the numbers in front) - save H and O until LAST! Check to make sure it is balanced. ...
... Balance the atoms of an element one at a time by adding coefficients (the numbers in front) - save H and O until LAST! Check to make sure it is balanced. ...
rocks and minerals quiz
... molecules. Molar heat capacity is the amount of heat needed to raise the temperature of 1 mole of a substance by 1o C. Example 5. Calculate the calories required to heat one kilogram of aluminum from 10oC to 70oC. ...
... molecules. Molar heat capacity is the amount of heat needed to raise the temperature of 1 mole of a substance by 1o C. Example 5. Calculate the calories required to heat one kilogram of aluminum from 10oC to 70oC. ...
Chemical Reactions
... • + sign separates molecules on the same side • The arrow is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen react to yield carbon dioxide” ...
... • + sign separates molecules on the same side • The arrow is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen react to yield carbon dioxide” ...
Chemical Reactions
... • + sign separates molecules on the same side • The arrow is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen react to yield carbon dioxide” ...
... • + sign separates molecules on the same side • The arrow is read as “yields” • Example C + O2 CO2 • This reads “carbon plus oxygen react to yield carbon dioxide” ...
Chapter 7
... In any chemical reaction, atoms are conserved… That is, the same number of atoms used in the reaction is the same number of atoms in the products. This is conservation of mass (or matter)…Matter is not created or destroyed, it just changes form. ...
... In any chemical reaction, atoms are conserved… That is, the same number of atoms used in the reaction is the same number of atoms in the products. This is conservation of mass (or matter)…Matter is not created or destroyed, it just changes form. ...
Chapter Five
... To balance chemical equations first count the number of each type of atom you have on both sides of the reaction. Identify any lone elements (as opposed to compounds) in the formulas; you will balance these last. From here, each equation requires its own logic; by trial and error, you should be able ...
... To balance chemical equations first count the number of each type of atom you have on both sides of the reaction. Identify any lone elements (as opposed to compounds) in the formulas; you will balance these last. From here, each equation requires its own logic; by trial and error, you should be able ...
Ch. 8 Notes (Chemical Reactions) Teacher 2010
... • There are two types of Nuclear reactions, ________________ – Fission reactions involve a heavy nucleus that will split into two or three pieces. – Fusion reactions involve two light nuclei that combine into a ...
... • There are two types of Nuclear reactions, ________________ – Fission reactions involve a heavy nucleus that will split into two or three pieces. – Fusion reactions involve two light nuclei that combine into a ...
Chapter 1 Student Notes
... All matter is composed of about 118 different kinds of atoms. These atoms can be physically mixed or chemically joined together to make up all kinds of matter. Atom the smallest unit of an element that maintains the properties of that element. Since matter exists in so many different forms, having ...
... All matter is composed of about 118 different kinds of atoms. These atoms can be physically mixed or chemically joined together to make up all kinds of matter. Atom the smallest unit of an element that maintains the properties of that element. Since matter exists in so many different forms, having ...
Chemistry Module 1- Basic Revision Notes 1.1a Atomic Structure 1.1
... 2FrCl (s) (Remember that the Halogens are diatomic! i.e. Cl2, Br2) ...
... 2FrCl (s) (Remember that the Halogens are diatomic! i.e. Cl2, Br2) ...
Honors Midterm - Stamford High School
... 2. Draw boxes around all the chemical formulas. Never, ever, change anything inside the boxes. Ever. Really. If you do, you're guaranteed to get the answer wrong. 3. Make an element inventory. How are you going to know if the equation is balanced if you don't actually make a list of how many of each ...
... 2. Draw boxes around all the chemical formulas. Never, ever, change anything inside the boxes. Ever. Really. If you do, you're guaranteed to get the answer wrong. 3. Make an element inventory. How are you going to know if the equation is balanced if you don't actually make a list of how many of each ...
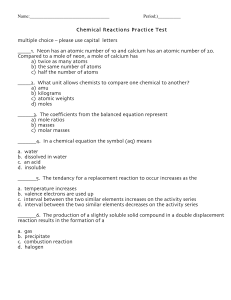
Chemical Reactions Practice Test
... _____1. Neon has an atomic number of 10 and calcium has an atomic number of 20. Compared to a mole of neon, a mole of calcium has a) twice as many atoms b) the same number of atoms c) half the number of atoms _____2. What unit allows chemists to compare one chemical to another? a) amu b) kilograms c ...
... _____1. Neon has an atomic number of 10 and calcium has an atomic number of 20. Compared to a mole of neon, a mole of calcium has a) twice as many atoms b) the same number of atoms c) half the number of atoms _____2. What unit allows chemists to compare one chemical to another? a) amu b) kilograms c ...
2016 Pre Course CHEMISTRY - Calday Grange Grammar School
... lubricant. Diamond and graphite both have high melting points. Explain each of these properties of diamond and graphite in terms of structure and bonding. Give one other difference in the properties of diamond and graphite. ...
... lubricant. Diamond and graphite both have high melting points. Explain each of these properties of diamond and graphite in terms of structure and bonding. Give one other difference in the properties of diamond and graphite. ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.