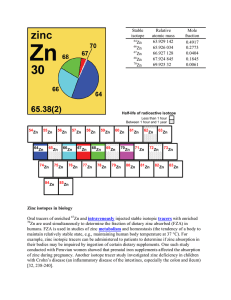
Zinc isotopes in biology Oral tracers of enriched Zn and
... maintain relatively stable state, e.g., maintaining human body temperature at 37 °C). For example, zinc isotopic tracers can be administered to patients to determine if zinc absorption in their bodies may be impaired by ingestion of certain dietary supplements. One such study conducted with Peruvian ...
... maintain relatively stable state, e.g., maintaining human body temperature at 37 °C). For example, zinc isotopic tracers can be administered to patients to determine if zinc absorption in their bodies may be impaired by ingestion of certain dietary supplements. One such study conducted with Peruvian ...
Print Off Slides for Class
... Rule for Addition and Subtraction Calculating with Numbers Written in Scientific Notation In order to add or subtract numbers written in scientific notation, you must express them with the same power of 10. ...
... Rule for Addition and Subtraction Calculating with Numbers Written in Scientific Notation In order to add or subtract numbers written in scientific notation, you must express them with the same power of 10. ...
Chapter 6 Chemical Reactions and Change
... In a chemical reaction,old bonds are broken and new bonds formed; atoms in the reactants are rearranged to form one or more different substances ...
... In a chemical reaction,old bonds are broken and new bonds formed; atoms in the reactants are rearranged to form one or more different substances ...
CHEMISTRY FALL FINAL PRACTICE 2016
... Write the nuclear notation for the most common isotope of chromium. ...
... Write the nuclear notation for the most common isotope of chromium. ...
Total Notes for chem - Catawba County Schools
... binding energy between quarks. One of the" GLUES OF THE UNIVERSE". Pion: The other "GLUE OF THE UNIVERSE", thought to be responsible for carrying binding energy between Gluons . ...
... binding energy between quarks. One of the" GLUES OF THE UNIVERSE". Pion: The other "GLUE OF THE UNIVERSE", thought to be responsible for carrying binding energy between Gluons . ...
Lecture 6
... A chemical equation gives the chemical formulas of the reactants on the left of the arrow and the products on the right. Since matter in a chemical reaction is conserved, the number of atoms you begin with must equal the number oand type you end up with. ...
... A chemical equation gives the chemical formulas of the reactants on the left of the arrow and the products on the right. Since matter in a chemical reaction is conserved, the number of atoms you begin with must equal the number oand type you end up with. ...
3_2: More Chemical Changes
... the classroom to help you during labs and quizzes • See Ms. B if you need paper, markers, etc. to take home for the night. • DUE MONDAY/TUESDAY ...
... the classroom to help you during labs and quizzes • See Ms. B if you need paper, markers, etc. to take home for the night. • DUE MONDAY/TUESDAY ...
Organic Chemistry and Medicine
... which is the major component of bacterial cell walls. - Bacterial cell wall synthesis is essential to growth, cell division (thus reproduction) and maintaining the cellular structure in bacteria. - Inhibition of PBPs leads to irregularities in cell wall structure such as elongation, lesions, loss of ...
... which is the major component of bacterial cell walls. - Bacterial cell wall synthesis is essential to growth, cell division (thus reproduction) and maintaining the cellular structure in bacteria. - Inhibition of PBPs leads to irregularities in cell wall structure such as elongation, lesions, loss of ...
worksheer format 11-12
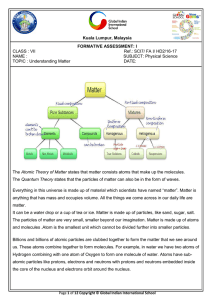
... Everything in this universe is made up of material which scientists have named “matter”. Matter is anything that has mass and occupies volume. All the things we come across in our daily life are matter. It can be a water drop or a cup of tea or ice. Matter is made up of particles, like sand, sugar, ...
... Everything in this universe is made up of material which scientists have named “matter”. Matter is anything that has mass and occupies volume. All the things we come across in our daily life are matter. It can be a water drop or a cup of tea or ice. Matter is made up of particles, like sand, sugar, ...
Sec. 12.3 Day 2
... the container wall. Each gas exerts a pressure proportional to its number of molecules in the container. The presence of other gas molecules does not change this fact. ...
... the container wall. Each gas exerts a pressure proportional to its number of molecules in the container. The presence of other gas molecules does not change this fact. ...
Document
... Quantities can only be added or subtracted if they have the same dimensions (units) Both sides of an algebraic equation or formula must have the same final units or it is invalid Formulas whose dimensions do not agree are invalid ...
... Quantities can only be added or subtracted if they have the same dimensions (units) Both sides of an algebraic equation or formula must have the same final units or it is invalid Formulas whose dimensions do not agree are invalid ...
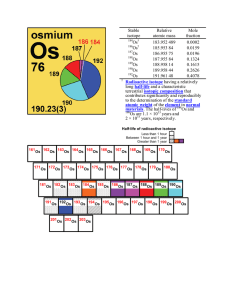
Stable isotope Relative atomic mass Mole fraction Os 183.952 489
... mole ratio n(187 Re)/n(186Os) are used for geochronology; for example, variations in these ratios have been used to determine the ages of the Earth, moon, and meteorites [299, 511, 524, 525]. Kirk et al. [526] measured rhenium-osmium isotopic abundances in gold and pyrites from conglomerates of the ...
... mole ratio n(187 Re)/n(186Os) are used for geochronology; for example, variations in these ratios have been used to determine the ages of the Earth, moon, and meteorites [299, 511, 524, 525]. Kirk et al. [526] measured rhenium-osmium isotopic abundances in gold and pyrites from conglomerates of the ...
Word Equations • a summary
... Use molecular building kits to look at the reaction of: Methane + CH4 ...
... Use molecular building kits to look at the reaction of: Methane + CH4 ...
Meeting no
... We want to find the number of moles of each element in order to determine the ratios of the elements and the formula. To make the calculation easy (i.e., let the percentages convert directly to grams), let's assume we have 100 g of vitamin C. If you are given mass percentages, always work with a hyp ...
... We want to find the number of moles of each element in order to determine the ratios of the elements and the formula. To make the calculation easy (i.e., let the percentages convert directly to grams), let's assume we have 100 g of vitamin C. If you are given mass percentages, always work with a hyp ...
IT IS ELEMENTARY - the OLLI at UCI Blog
... and animal origin • These elements or their very simple compounds can kill—most commonly by interfering with cellular access to oxygen • Nitrogen N2 • Carbon dioxide CO2 • Carbon monoxide CO • Hydrogen cyanide HCN ...
... and animal origin • These elements or their very simple compounds can kill—most commonly by interfering with cellular access to oxygen • Nitrogen N2 • Carbon dioxide CO2 • Carbon monoxide CO • Hydrogen cyanide HCN ...
AP Chemistry Summer Assignment
... For those students who have just taken Chemistry 1, much of the material in the summer packet will be familiar to you. For those students who have not taken Chemistry for a while the problems will help you rebuild a foundation in chemistry and insure all students are on a relatively even plane. It w ...
... For those students who have just taken Chemistry 1, much of the material in the summer packet will be familiar to you. For those students who have not taken Chemistry for a while the problems will help you rebuild a foundation in chemistry and insure all students are on a relatively even plane. It w ...
Name_____________________________________ Chemistry
... 4. a. Define Density & write its formula. ...
... 4. a. Define Density & write its formula. ...
History of chemistry

The history of chemistry represents a time span from ancient history to the present. By 1000 BC, civilizations used technologies that would eventually form the basis to the various branches of chemistry. Examples include extracting metals from ores, making pottery and glazes, fermenting beer and wine, extracting chemicals from plants for medicine and perfume, rendering fat into soap, making glass, and making alloys like bronze.The protoscience of chemistry, alchemy, was unsuccessful in explaining the nature of matter and its transformations. However, by performing experiments and recording the results, alchemists set the stage for modern chemistry. The distinction began to emerge when a clear differentiation was made between chemistry and alchemy by Robert Boyle in his work The Sceptical Chymist (1661). While both alchemy and chemistry are concerned with matter and its transformations, chemists are seen as applying scientific method to their work.Chemistry is considered to have become an established science with the work of Antoine Lavoisier, who developed a law of conservation of mass that demanded careful measurement and quantitative observations of chemical phenomena. The history of chemistry is intertwined with the history of thermodynamics, especially through the work of Willard Gibbs.