2012 Coaches Institute Presentation
... Assume AgCrO4 dissociates completely in water at 25oC. [Ag+] = 1.3 x 10-4 AgCrO4(s) ⇔ 2Ag+(aq) + CrO4-2(aq) Ksp = [Ag+]2[CrO4-2] [CrO4-2] = 1.3 x 10-4 mol Ag+ x 1 mol CrO4-2 ...
... Assume AgCrO4 dissociates completely in water at 25oC. [Ag+] = 1.3 x 10-4 AgCrO4(s) ⇔ 2Ag+(aq) + CrO4-2(aq) Ksp = [Ag+]2[CrO4-2] [CrO4-2] = 1.3 x 10-4 mol Ag+ x 1 mol CrO4-2 ...
ChLM Final Review Name: Period: Base Knowledge 1. Classify the
... 23. Use the periodic table to identify the element in period 3, group 17. What do you know about this element? (What is the name of its group? How many valence electrons does it have? ...
... 23. Use the periodic table to identify the element in period 3, group 17. What do you know about this element? (What is the name of its group? How many valence electrons does it have? ...
Chemistry Test Review - Greenslime Home Page
... 6. How many protons are in one molecule of NH4? Two molecules? a. 11 protons in one molecule b. 22 protons in 2 molecules 7. Describe the chemical formula: 4NaHCO3 a. 4 molecules of a compound containing 1 atom of the element Sodium, 1 atom of the element Hydrogen, 1 atom of the element Carbon and 3 ...
... 6. How many protons are in one molecule of NH4? Two molecules? a. 11 protons in one molecule b. 22 protons in 2 molecules 7. Describe the chemical formula: 4NaHCO3 a. 4 molecules of a compound containing 1 atom of the element Sodium, 1 atom of the element Hydrogen, 1 atom of the element Carbon and 3 ...
Single Covalent bonds
... A prefix is added to the name of the first element in the formula ONLY if more than one atom of it is present. A prefix is ALWAYS added to the name of the second element in the formula The second element will use the form of its name ending ...
... A prefix is added to the name of the first element in the formula ONLY if more than one atom of it is present. A prefix is ALWAYS added to the name of the second element in the formula The second element will use the form of its name ending ...
Single Covalent bonds
... A prefix is added to the name of the first element in the formula ONLY if more than one atom of it is present. A prefix is ALWAYS added to the name of the second element in the formula The second element will use the form of its name ending ...
... A prefix is added to the name of the first element in the formula ONLY if more than one atom of it is present. A prefix is ALWAYS added to the name of the second element in the formula The second element will use the form of its name ending ...
Bonding Web Practice Trupia - Trupia
... Bromine is the only liquid nonmetallic element at room temperature. It is a heavy, mobile, reddish-brown liquid, volatilizing readily at room temperature to a red vapor with a strong disagreeable odor, resembling chlorine, and having a very irritating effect on the eyes and throat; it is readily sol ...
... Bromine is the only liquid nonmetallic element at room temperature. It is a heavy, mobile, reddish-brown liquid, volatilizing readily at room temperature to a red vapor with a strong disagreeable odor, resembling chlorine, and having a very irritating effect on the eyes and throat; it is readily sol ...
An element`s properties depend on the structure of its atoms
... • A cation is a positively charged ion • An anion is a negatively charged ion • An ionic bond is an attraction between an anion and a cation • Compounds formed by ionic bonds are called ionic compounds, or salts • Salts, such as sodium chloride (table salt), are often found in nature as crystals ...
... • A cation is a positively charged ion • An anion is a negatively charged ion • An ionic bond is an attraction between an anion and a cation • Compounds formed by ionic bonds are called ionic compounds, or salts • Salts, such as sodium chloride (table salt), are often found in nature as crystals ...
CHEMISTRY FINAL EXAM REVIEW SHEET
... A monatomic ion has an oxidation number equal to its charge. Hydrogen is usually +1. Oxygen is usually –2. In a compound, the more electronegative element is given an oxidation number equal to its usual ionic charge. The sum of the oxidation numbers must equal the overall charge on the compound or i ...
... A monatomic ion has an oxidation number equal to its charge. Hydrogen is usually +1. Oxygen is usually –2. In a compound, the more electronegative element is given an oxidation number equal to its usual ionic charge. The sum of the oxidation numbers must equal the overall charge on the compound or i ...
CHM 101 - Academic Computer Center
... Oxygen can form either covalent or ionic bonds. Explain the nature of each bond, the conditions under which each forms and the type of substances in each bond. ...
... Oxygen can form either covalent or ionic bonds. Explain the nature of each bond, the conditions under which each forms and the type of substances in each bond. ...
Organic Chemistry William H. Brown Christopher S. Foote
... a complete valence shell • show a bonding pair of electrons as a single line • show a nonbonding pair of electrons as a pair of dots • in a single bond atoms share one pair of electrons, in a double bond they share two pairs of electrons, and in a triple bond they share three pairs of electrons ...
... a complete valence shell • show a bonding pair of electrons as a single line • show a nonbonding pair of electrons as a pair of dots • in a single bond atoms share one pair of electrons, in a double bond they share two pairs of electrons, and in a triple bond they share three pairs of electrons ...
Chapter 2 Chemical context of Life
... The electrons of an atom have potential energy because of how they are arranged in relation to the nucleus. Electrons are attracted by the positive nucleus. It takes energy to move electrons farther away from the nucleus. Electrons have fixed amounts of potential energy that correspond to a position ...
... The electrons of an atom have potential energy because of how they are arranged in relation to the nucleus. Electrons are attracted by the positive nucleus. It takes energy to move electrons farther away from the nucleus. Electrons have fixed amounts of potential energy that correspond to a position ...
ch-4-earth-chemistry
... different number of neutrons or atomic mass would not change the atom into a different element. It just changes the atomic mass. ...
... different number of neutrons or atomic mass would not change the atom into a different element. It just changes the atomic mass. ...
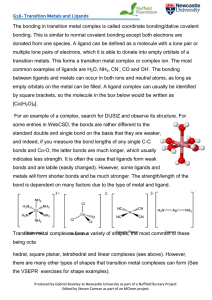
The bonding in transition metal complex is called coordinate
... For an example of a complex, search for DIJSIZ and observe its structure. For some entries in WebCSD, the bonds are rather different to the standard double and single bond on the basis that they are weaker, and indeed, if you measure the bond lengths of any single C-C bonds and Co-O, the latter bond ...
... For an example of a complex, search for DIJSIZ and observe its structure. For some entries in WebCSD, the bonds are rather different to the standard double and single bond on the basis that they are weaker, and indeed, if you measure the bond lengths of any single C-C bonds and Co-O, the latter bond ...
Chapter8
... electrons to satisfy the octet rule (or duet rule for hydrogen). •The central atom is usually written first in the formula [the central atom is usually the least electronegative atom, exceptions in H2O, and NH3 where O and N are the central atoms] •Hydrogen is never the central atom ...
... electrons to satisfy the octet rule (or duet rule for hydrogen). •The central atom is usually written first in the formula [the central atom is usually the least electronegative atom, exceptions in H2O, and NH3 where O and N are the central atoms] •Hydrogen is never the central atom ...
File
... its own unique properties is formed. Good evidence is when a new gas is formed; heat or light is produced; a precipitate is formed; rusting/tarnishing 38. Was the zinc and hydrochloric acid combination a chemical reaction? What were some indicators? Yes because a new gas was formed 39. If I combined ...
... its own unique properties is formed. Good evidence is when a new gas is formed; heat or light is produced; a precipitate is formed; rusting/tarnishing 38. Was the zinc and hydrochloric acid combination a chemical reaction? What were some indicators? Yes because a new gas was formed 39. If I combined ...
Bio1A Unit 1-1 Chem Notes File
... • Negative feedback means that as more of a product accumulates, the process that creates it slows and less of the product is produced • Positive feedback means that as more of a product accumulates, the process that creates it speeds up and more of the product is produced ...
... • Negative feedback means that as more of a product accumulates, the process that creates it slows and less of the product is produced • Positive feedback means that as more of a product accumulates, the process that creates it speeds up and more of the product is produced ...
Bond
... including its role in nature, depends primarily on its molecular structure, or shape. Molecular shape contributes toward determining a compound’s boiling point, freezing point, viscosity, solubility, types of reactions it can participate in, and a host of other physical and chemical properties. The ...
... including its role in nature, depends primarily on its molecular structure, or shape. Molecular shape contributes toward determining a compound’s boiling point, freezing point, viscosity, solubility, types of reactions it can participate in, and a host of other physical and chemical properties. The ...
Compounds: SOL Review #3 Name: Ionic and Covalent Bonds 1
... 31) PreIB only: Why are molecules A and B polar, but molecule C is nonpolar? polar polar ...
... 31) PreIB only: Why are molecules A and B polar, but molecule C is nonpolar? polar polar ...
Click here to Ch 06.2 Covalent Bonding_Lewis Structures
... electrons, and for those that can fit more than eight electrons, into their outermost orbital. • Hydrogen forms bonds in which it is surrounded by only two electrons. • Boron has just three valence electrons, so it tends to form bonds in which it is surrounded by six electrons. ...
... electrons, and for those that can fit more than eight electrons, into their outermost orbital. • Hydrogen forms bonds in which it is surrounded by only two electrons. • Boron has just three valence electrons, so it tends to form bonds in which it is surrounded by six electrons. ...
Chapter 5 Notes: The Structure of Matter
... This makes its charge 1+ because there are more + charges ...
... This makes its charge 1+ because there are more + charges ...
Lecture 3 Chemistry
... Number of electrons in outer shell determines bonding properties chemical behavior ...
... Number of electrons in outer shell determines bonding properties chemical behavior ...
sample paper chemistry clas xi set 3
... whereas in Mg atom, due to small size, large amount ofenergy is required ...
... whereas in Mg atom, due to small size, large amount ofenergy is required ...
apch08 lecture 1 - Success in AP Chemistry
... HIGH ionization energy so they will GAIN electrons to form anions, negative ions. Both a chloride ion and the argon atom have an octet of electrons in their highest occupied energy levels. ...
... HIGH ionization energy so they will GAIN electrons to form anions, negative ions. Both a chloride ion and the argon atom have an octet of electrons in their highest occupied energy levels. ...