Atoms and Elements Notes
... 1. Physical Change- A change in form that does not result in a new substance. Ex. Sugar dissolves in water or any state change. 2. Chemical Change- A change/reaction that creates a new substance. An indication this is happening is when you see a color change, heat, light, smoke/gas and a new byprodu ...
... 1. Physical Change- A change in form that does not result in a new substance. Ex. Sugar dissolves in water or any state change. 2. Chemical Change- A change/reaction that creates a new substance. An indication this is happening is when you see a color change, heat, light, smoke/gas and a new byprodu ...
CHEMISTRY
... Smallest unit nucleus: center/core is most of the mass of the atom a. protons: + charge ...
... Smallest unit nucleus: center/core is most of the mass of the atom a. protons: + charge ...
Minerals * Chemistry Review
... • The number of protons plus neutrons gives the atom its atomic mass • All atoms of a given element have the same number of protons ...
... • The number of protons plus neutrons gives the atom its atomic mass • All atoms of a given element have the same number of protons ...
chemical bond
... of (+) and (-) ions held together by ionic bonding. An ionic compound transfers electron so that the compound becomes neutral. Metal + non-metal = ionic bonding (Overhead) ...
... of (+) and (-) ions held together by ionic bonding. An ionic compound transfers electron so that the compound becomes neutral. Metal + non-metal = ionic bonding (Overhead) ...
2 Types of Chemical Bonds
... • A chemical bond is formed when atoms of elements change the number of valence electrons they have to get 8 or 2 • A chemical bond combines elements together to form a compound! ...
... • A chemical bond is formed when atoms of elements change the number of valence electrons they have to get 8 or 2 • A chemical bond combines elements together to form a compound! ...
TEST REVIEW S Valence Electrons TEST REVIEW SHEET 2017
... NOTE: If an element has <4 valence electrons it will give them away during an ionic bond and become a positive ion. If >4, it will take them and become a negative ion For the most part…. metals will give away their valence electrons and nonmetals will take enough valence electrons to fill their oute ...
... NOTE: If an element has <4 valence electrons it will give them away during an ionic bond and become a positive ion. If >4, it will take them and become a negative ion For the most part…. metals will give away their valence electrons and nonmetals will take enough valence electrons to fill their oute ...
1. I can define valence electron and use the periodic
... #2. I can make a Lewis dot drawing of an element. 5. Make Lewis Dot structures for all the elements listed above (a-j). #3. I can explain how valence electrons are related to chemical reactivity. 6. Which elements react violently with water? 7. Which anions are most reactive? 8. Why are these atoms ...
... #2. I can make a Lewis dot drawing of an element. 5. Make Lewis Dot structures for all the elements listed above (a-j). #3. I can explain how valence electrons are related to chemical reactivity. 6. Which elements react violently with water? 7. Which anions are most reactive? 8. Why are these atoms ...
Chapter 6: Chemical Bonding
... bonded atoms at their minimum potential energy. AKA average distance between two bonded atoms. • Bond Energy – The energy required to break a chemical bond or form neutral isolated atoms. ...
... bonded atoms at their minimum potential energy. AKA average distance between two bonded atoms. • Bond Energy – The energy required to break a chemical bond or form neutral isolated atoms. ...
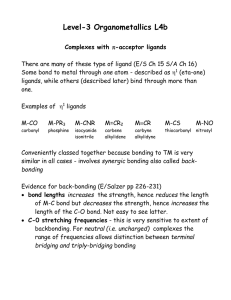
lect4b
... Level-3 Organometallics L4b Complexes with -acceptor ligands There are many of these type of ligand (E/S Ch 15 S/A Ch 16) Some bond to metal through one atom - described as 1 (eta-one) ligands, while others (described later) bind through more than one. Examples of 1 ligands M-CO ...
... Level-3 Organometallics L4b Complexes with -acceptor ligands There are many of these type of ligand (E/S Ch 15 S/A Ch 16) Some bond to metal through one atom - described as 1 (eta-one) ligands, while others (described later) bind through more than one. Examples of 1 ligands M-CO ...
Advanced Chemistry Midterm
... 23. What are the electronegativity difference ranges for nonpolar bonds? For polar bonds? For ionic bonds? ...
... 23. What are the electronegativity difference ranges for nonpolar bonds? For polar bonds? For ionic bonds? ...
Draw atomic models showing the appropriate number of electrons
... 7. Any electron in the outermost occupied shell of an atom 8. Atoms with a positive or negative charge due to loss or gain of electrons ...
... 7. Any electron in the outermost occupied shell of an atom 8. Atoms with a positive or negative charge due to loss or gain of electrons ...
Chem 101A Exam 4 Concepts Chapter 7 – Modern Atomic Theory
... Ionic bond, coulomb’s law and lattice energy (they all relate) Ionic radius trends (atom vs ion, and compare isoelectronic series) Bond energies to calculate Hrxn (Ebonds broken – Ebonds formed) Lewis structures predict which atoms bond to which and nonbonding electrons (lone pair) 2 valenc ...
... Ionic bond, coulomb’s law and lattice energy (they all relate) Ionic radius trends (atom vs ion, and compare isoelectronic series) Bond energies to calculate Hrxn (Ebonds broken – Ebonds formed) Lewis structures predict which atoms bond to which and nonbonding electrons (lone pair) 2 valenc ...
Chapter 8: Chemical Bonding
... Hence: atoms tend to be surrounded by 8 valence e- - this is the reason that group 1 atoms form +1 ions, group 6 atoms form -2 ions, etc ...
... Hence: atoms tend to be surrounded by 8 valence e- - this is the reason that group 1 atoms form +1 ions, group 6 atoms form -2 ions, etc ...
Biol 1441
... Electronegativity: is the attraction of a particular kind of atom for the electrons of a covalent bond Nonpolar covalent bond: the electrons of the bond are shared equally. Ex: N2 Polar covalent bond: the electrons of the bond are not shared equally. Ex: HCl Ionic Bonds: Two atoms are so unequal in ...
... Electronegativity: is the attraction of a particular kind of atom for the electrons of a covalent bond Nonpolar covalent bond: the electrons of the bond are shared equally. Ex: N2 Polar covalent bond: the electrons of the bond are not shared equally. Ex: HCl Ionic Bonds: Two atoms are so unequal in ...
ionic and covalent bonds
... Octet Rule: atoms want 8 electrons in the valence (outer) energy level Ionic Bond: bond in which one or more electrons from one atom are removed and attached to another atom. This creates positive and negative ions which attract each other. (Acids, bases, salts do this): ...
... Octet Rule: atoms want 8 electrons in the valence (outer) energy level Ionic Bond: bond in which one or more electrons from one atom are removed and attached to another atom. This creates positive and negative ions which attract each other. (Acids, bases, salts do this): ...