Writing Formulas
... Writing Ionic Formulas When writing the chemical formula for ionic compounds put the cation first followed by the anion and use subscripts to indicate the number of each ion present. Remember the algebraic sum of the ions' oxidation numbers must equal zero. (Balance) Learn the polyatomic ions. ...
... Writing Ionic Formulas When writing the chemical formula for ionic compounds put the cation first followed by the anion and use subscripts to indicate the number of each ion present. Remember the algebraic sum of the ions' oxidation numbers must equal zero. (Balance) Learn the polyatomic ions. ...
Chapter 8
... Compounds usually have new or different properties than the elements that make up the compound ...
... Compounds usually have new or different properties than the elements that make up the compound ...
Sample Exam 1 Key
... a) All matter is composed of indivisible atoms. b) Atoms of different elements differ in size and properties. c) Isotopes of the same element have different chemical properties. d) A chemical reaction occurs when atoms recombine in whole number ratios. 3. Which of the following has the longest wavel ...
... a) All matter is composed of indivisible atoms. b) Atoms of different elements differ in size and properties. c) Isotopes of the same element have different chemical properties. d) A chemical reaction occurs when atoms recombine in whole number ratios. 3. Which of the following has the longest wavel ...
Chapter 4 Chemical Foundations: Elements, Atoms, and Ions
... – Atoms of one element cannot change into atoms of another element. • Cannot turn lead into gold by a chemical reaction ...
... – Atoms of one element cannot change into atoms of another element. • Cannot turn lead into gold by a chemical reaction ...
4. bonding - New Hartford Central Schools
... Elements with more than one positive oxidation number (Transition Metals) (This is called the Stock System) When the oxidation number varies we us a Roman numeral in parentheses to indicate the charge. Roman number is used for the positive element only!!! ...
... Elements with more than one positive oxidation number (Transition Metals) (This is called the Stock System) When the oxidation number varies we us a Roman numeral in parentheses to indicate the charge. Roman number is used for the positive element only!!! ...
Slide 1
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
... The symbol for the magnetic quantum number is m which defines the orbital. m = - , (- + 1), (- +2), .....0, ......., ( -2), ( -1), The last quantum number is the spin quantum number which has the symbol m s which characterizes the single electron. The spin quantum number only has two pos ...
FINAL EXAM Review Sheet / Study Guide Honors Chemistry
... 33) Write the name of each of the following compounds. a) ________________________ AgNO3 ...
... 33) Write the name of each of the following compounds. a) ________________________ AgNO3 ...
CHE 1401 - Fall 2013 - Chapter 7 Homework 7 (Chapter 7: Periodic
... A) alkali metals have lower densities B) alkali metals have greater electron affinities C) alkali metals have lower ionization energies D) alkali metals have lower melting points E) alkali metals are not more reactive than alkaline earth metals ...
... A) alkali metals have lower densities B) alkali metals have greater electron affinities C) alkali metals have lower ionization energies D) alkali metals have lower melting points E) alkali metals are not more reactive than alkaline earth metals ...
AP Chemistry 2013 Semester 1 Final Exam Review Problems
... b. What is the molecular formula of this substance? c. Draw the Lewis structure of the molecule using the fact that the Cl atoms bond to a single C atom, there is a C-C bond, and two C-O bonds in the compound. 17. Draw the Lewis structures for BH3 and NH3. a. What is the bond angle around the centra ...
... b. What is the molecular formula of this substance? c. Draw the Lewis structure of the molecule using the fact that the Cl atoms bond to a single C atom, there is a C-C bond, and two C-O bonds in the compound. 17. Draw the Lewis structures for BH3 and NH3. a. What is the bond angle around the centra ...
Nature of Molecules and Water
... – Na atom loses an electron to become Na+ – Cl atom gains an electron to become Cl– – Opposite charges attract so that Na+ and Cl– remain associated as an ionic compound • Electrical attraction of water molecules can disrupt forces holding ions together ...
... – Na atom loses an electron to become Na+ – Cl atom gains an electron to become Cl– – Opposite charges attract so that Na+ and Cl– remain associated as an ionic compound • Electrical attraction of water molecules can disrupt forces holding ions together ...
CHEM 1305 - HCC Learning Web
... PART II – Show your work: (8 points each) 21a. Element X has natural isotopes; X-63 (62.940amu) and X-65 (64.928amu). Calculate the atomic mass of element X given the abundance of X-63 is 69.17% b. Which element corresponds to each of the following electron configuration? i. 1S2 2S2 2P5 ii. 1S2 2S2 ...
... PART II – Show your work: (8 points each) 21a. Element X has natural isotopes; X-63 (62.940amu) and X-65 (64.928amu). Calculate the atomic mass of element X given the abundance of X-63 is 69.17% b. Which element corresponds to each of the following electron configuration? i. 1S2 2S2 2P5 ii. 1S2 2S2 ...
Chemistry Midterm Review 2006
... 3. Identify which ones have dipole-dipole forces? PBr3, N2, CF4, HBr, H2O 4. Identify which ones have London dispersion forces? , N2, CF4, HBr, SO2 5. Identify which ones have hydrogen bonding? HCl,, H2, HBr, H2O, CH4 6. Define the physical properties of Viscosity, Surface Tension, Boiling Point and ...
... 3. Identify which ones have dipole-dipole forces? PBr3, N2, CF4, HBr, H2O 4. Identify which ones have London dispersion forces? , N2, CF4, HBr, SO2 5. Identify which ones have hydrogen bonding? HCl,, H2, HBr, H2O, CH4 6. Define the physical properties of Viscosity, Surface Tension, Boiling Point and ...
Chemistry Outcomes - hrsbstaff.ednet.ns.ca
... Explain the hydrogen line spectrum in terms of Bohr Model of the atom State two differences between the Bohr model and the quantum mechanical model of the atom Draw an energy level diagram for a given atom Define valence shell and valence electrons Label the sublevels on an energy level diagram with ...
... Explain the hydrogen line spectrum in terms of Bohr Model of the atom State two differences between the Bohr model and the quantum mechanical model of the atom Draw an energy level diagram for a given atom Define valence shell and valence electrons Label the sublevels on an energy level diagram with ...
Honors Biology Chapter 2 Power Point
... If it could as easily lose or gain e-, then it will probably share them. It will form a covalent bond. ...
... If it could as easily lose or gain e-, then it will probably share them. It will form a covalent bond. ...
Chemistry 11 – Course Outcomes
... Explain the hydrogen line spectrum in terms of Bohr Model of the atom State two differences between the Bohr model and the quantum mechanical model of the atom Draw an energy level diagram for a given atom Define valence shell and valence electrons Label the sublevels on an energy level diagram with ...
... Explain the hydrogen line spectrum in terms of Bohr Model of the atom State two differences between the Bohr model and the quantum mechanical model of the atom Draw an energy level diagram for a given atom Define valence shell and valence electrons Label the sublevels on an energy level diagram with ...
Elements Elements (cont.) Elements (cont.)
... • A compound is a distinct substance that is composed d off atoms t off two t or more elements. l t • Compounds are identified by the number and type of each atom in the simplest unit of the compound. – Molecules or ions ...
... • A compound is a distinct substance that is composed d off atoms t off two t or more elements. l t • Compounds are identified by the number and type of each atom in the simplest unit of the compound. – Molecules or ions ...
Practice Exam 2 - Department of Chemistry and Biochemistry
... When 16 g of methane is burned according to the equation CH4 + 2O2 CO2 + 2H2O , you experimentally measure you have produced 32 g of water. What is the percent yield for this reaction? A. 79% B. 85% C. 95% D. 62% E. 89% ...
... When 16 g of methane is burned according to the equation CH4 + 2O2 CO2 + 2H2O , you experimentally measure you have produced 32 g of water. What is the percent yield for this reaction? A. 79% B. 85% C. 95% D. 62% E. 89% ...
Chapter 8 Study Guide
... a. Chemists discovered that if two or more different compounds are composed of the same elements, the ratio of the masses of the second element is always a ratio of small whole numbers. This example illustrates the law of multiple proportions ...
... a. Chemists discovered that if two or more different compounds are composed of the same elements, the ratio of the masses of the second element is always a ratio of small whole numbers. This example illustrates the law of multiple proportions ...
The Periodic Table
... Electron affinity does not change greatly as we move down a group. Electron affinity should become more positive (less energy released). Reason: Moving down a group the average distance between the added electron and the nucleus steadily increases, causing the electron-nucleus attraction to decrease ...
... Electron affinity does not change greatly as we move down a group. Electron affinity should become more positive (less energy released). Reason: Moving down a group the average distance between the added electron and the nucleus steadily increases, causing the electron-nucleus attraction to decrease ...
Sem 1 Final
... Subatomic particles • Which subatomic particles is the most responsible for the chemical properties of the element? ...
... Subatomic particles • Which subatomic particles is the most responsible for the chemical properties of the element? ...
SCH 3U - othsmath
... decreases the ENC (even though the number of protons in the nucleus is increasing) and causes the atomic radius to increase. Thus, the further down the group we go, the less strongly the valence electrons are held and the less likely the atom will pull electrons toward itself in a bond, so the lower ...
... decreases the ENC (even though the number of protons in the nucleus is increasing) and causes the atomic radius to increase. Thus, the further down the group we go, the less strongly the valence electrons are held and the less likely the atom will pull electrons toward itself in a bond, so the lower ...
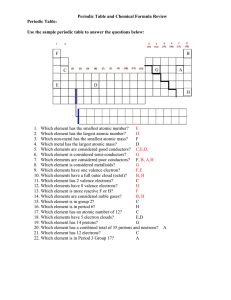
Periodic Table Review Key
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
... 9. Which elements have one valence electron? F,E 10. Which elements have a full outer cloud (octet)? B, H 11. Which element has 2 valence electrons? C 12. Which elements have 8 valence electrons? H 13. Which element is more reactive F or B? F 14. Which elements are considered noble gases? B, H 15. W ...
File - Mr. Holz`s Website
... Ionic Bond – Transfer of electrons to create a bond between two ions that are attracted by opposite charges Covalent Bond – Bond that forms when electrons are shared between atoms Ion – Charged atoms that form from ionic bonds; atoms in which the number of electrons does not equal the number of prot ...
... Ionic Bond – Transfer of electrons to create a bond between two ions that are attracted by opposite charges Covalent Bond – Bond that forms when electrons are shared between atoms Ion – Charged atoms that form from ionic bonds; atoms in which the number of electrons does not equal the number of prot ...
2. Chemistry of Living Things Outline
... When electrons are transferred from one atom to another, _______ atoms become electrically ____________. The atom that __________ electrons becomes ____________ charged. The atom that __________ electrons becomes ____________ charged. The ions with ___________ electrical charges are ____________ to ...
... When electrons are transferred from one atom to another, _______ atoms become electrically ____________. The atom that __________ electrons becomes ____________ charged. The atom that __________ electrons becomes ____________ charged. The ions with ___________ electrical charges are ____________ to ...
Electronegativity

Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. The term ""electronegativity"" was introduced by Jöns Jacob Berzelius in 1811,though the concept was known even before that and was studied by many chemists including Avogadro.In spite of its long history, an accurate scale of electronegativity had to wait till 1932, when Linus Pauling proposed an electronegativity scale, which depends on bond energies, as a development of valence bond theory. It has been shown to correlate with a number of other chemical properties. Electronegativity cannot be directly measured and must be calculated from other atomic or molecular properties. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of the electronegativity, all methods show the same periodic trends between elements. The most commonly used method of calculation is that originally proposed by Linus Pauling. This gives a dimensionless quantity, commonly referred to as the Pauling scale, on a relative scale running from around 0.7 to 3.98 (hydrogen = 2.20). When other methods of calculation are used, it is conventional (although not obligatory) to quote the results on a scale that covers the same range of numerical values: this is known as an electronegativity in Pauling units. As it is usually calculated, electronegativity is not a property of an atom alone, but rather a property of an atom in a molecule. Properties of a free atom include ionization energy and electron affinity. It is to be expected that the electronegativity of an element will vary with its chemical environment, but it is usually considered to be a transferable property, that is to say that similar values will be valid in a variety of situations.On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more ""pull"" it will have on electrons) and the number/location of other electrons present in the atomic shells (the more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result the less positive charge they will experience—both because of their increased distance from the nucleus, and because the other electrons in the lower energy core orbitals will act to shield the valence electrons from the positively charged nucleus).The opposite of electronegativity is electropositivity: a measure of an element's ability to donate electrons.Caesium is the least electronegative element in the periodic table (=0.79), while fluorine is most electronegative (=3.98). (Francium and caesium were originally assigned both assigned 0.7; caesium's value was later refined to 0.79, but no experimental data allows a similar refinement for francium. However, francium's ionization energy is known to be slightly higher than caesium's, in accordance with the relativistic stabilization of the 7s orbital, and this in turn implies that caesium is in fact more electronegative than francium.)