Chemistry of Living Things Outline
... Living and nonliving things are made up of tiny units called ________. The center core is called the _____________. The nucleus is made up of particles called __________ and ___________ _____________ have a positive charge (+1) and _____________ have no electrical charge (0). Negatively charged pa ...
... Living and nonliving things are made up of tiny units called ________. The center core is called the _____________. The nucleus is made up of particles called __________ and ___________ _____________ have a positive charge (+1) and _____________ have no electrical charge (0). Negatively charged pa ...
SOL Essential Knowledge
... 5. Loss of electrons from neutral atoms results in the formation of an ion with a positive charge (cation). 6. Gain of electrons by a neutral atom results in the formation of an ion with negative charge (anion). F. State and identify the location of the following on the periodic table: alkali metals ...
... 5. Loss of electrons from neutral atoms results in the formation of an ion with a positive charge (cation). 6. Gain of electrons by a neutral atom results in the formation of an ion with negative charge (anion). F. State and identify the location of the following on the periodic table: alkali metals ...
Chemistry Study Guide What is matter made of? Matter is anything
... The number of shells an atom has depends upon its number of electrons. Each shell must have its full number of electrons before a new shell starts. The outer shell of most electrons is not full. Only the atoms of the elements of Group 18 have full outer shells. ● Atoms of most metals have fewer than ...
... The number of shells an atom has depends upon its number of electrons. Each shell must have its full number of electrons before a new shell starts. The outer shell of most electrons is not full. Only the atoms of the elements of Group 18 have full outer shells. ● Atoms of most metals have fewer than ...
Chemical Bonding I
... nuclei of the bonded atoms. • As with bond energies, these are averages since there are slight variaGons according to the molecular structure. • The next few slides give some typical values. • N ...
... nuclei of the bonded atoms. • As with bond energies, these are averages since there are slight variaGons according to the molecular structure. • The next few slides give some typical values. • N ...
Chem Final Study Guide Energy How much heat energy must be
... a) Proton donor- acid. Proton acceptor- base. Water is amphoteric… can be either an acid or base, depending on what it is paired with. Use the following Solubility Graph to answer questions 73) Which compound is least soluble in water at 40.°C? most soluble? a) Least soluble- SO2 Most Soluble- KI 74 ...
... a) Proton donor- acid. Proton acceptor- base. Water is amphoteric… can be either an acid or base, depending on what it is paired with. Use the following Solubility Graph to answer questions 73) Which compound is least soluble in water at 40.°C? most soluble? a) Least soluble- SO2 Most Soluble- KI 74 ...
effective nuclear charge
... increase in size down the Group atomic radii of transition metals roughly the same size across the d block ◦ must less difference than across main group elements ◦ valence shell ns2, not the d electrons ◦ effective nuclear charge on the ns2 electrons approximately the same ...
... increase in size down the Group atomic radii of transition metals roughly the same size across the d block ◦ must less difference than across main group elements ◦ valence shell ns2, not the d electrons ◦ effective nuclear charge on the ns2 electrons approximately the same ...
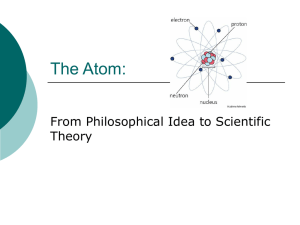
The Atom - Williamstown Independent Schools
... If two or more different compounds are composed of the same two elements then ratios of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers. ...
... If two or more different compounds are composed of the same two elements then ratios of the masses of the second element combined with a certain mass of the first element is always a ratio of small whole numbers. ...
APS Science Curriculum Unit Planner
... The smallest unique particle of matter is an atom and atoms can combine physically and chemically. Correlations Unifying Understanding ...
... The smallest unique particle of matter is an atom and atoms can combine physically and chemically. Correlations Unifying Understanding ...
Final
... electronegativity (table will be provided) Given a Lewis structure, be able to: identify the shape identify the hybridization of the central atom draw the molecule with the correct shape give the bonding description for all bonds within the structure Be able to determine whether a bond is nonpolar c ...
... electronegativity (table will be provided) Given a Lewis structure, be able to: identify the shape identify the hybridization of the central atom draw the molecule with the correct shape give the bonding description for all bonds within the structure Be able to determine whether a bond is nonpolar c ...
Ch 8 AP Practice
... (a) Write the equation for the ionization of atomic fluorine that requires 1,681.0 kJ mol-1. (b) Account for the fact that the first ionization energy of atomic fluorine is greater than that of atomic oxygen. (You must discuss both atoms in your response.) (c) Predict whether the first ionization en ...
... (a) Write the equation for the ionization of atomic fluorine that requires 1,681.0 kJ mol-1. (b) Account for the fact that the first ionization energy of atomic fluorine is greater than that of atomic oxygen. (You must discuss both atoms in your response.) (c) Predict whether the first ionization en ...
2014MSC(ORGANIC(CHEMISTRY!
... ! The!hydrogen!is!taken!off!due!to!the!nucleophile!(the!carbons!in!the!double!bond!–!electron! rich!due!to!the!pie!orbitals!above!and!below!the!double!bond)!attacking!it!with!electrons.! These!electrons!then!attach!to!the!oxygen,!replacing!the!hydrogen!and!giving!the!water!its! normal,!neutral!charg ...
... ! The!hydrogen!is!taken!off!due!to!the!nucleophile!(the!carbons!in!the!double!bond!–!electron! rich!due!to!the!pie!orbitals!above!and!below!the!double!bond)!attacking!it!with!electrons.! These!electrons!then!attach!to!the!oxygen,!replacing!the!hydrogen!and!giving!the!water!its! normal,!neutral!charg ...
Sub Unit Plan 1 Chem Periodic Table
... have the same atomic number. Elements cannot be broken down by chemical change. 3.1v Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases. 3.1w Elements can be differentiated by physical properties. ...
... have the same atomic number. Elements cannot be broken down by chemical change. 3.1v Elements can be classified by their properties and located on the Periodic Table as metals, nonmetals, metalloids (B, Si, Ge, As, Sb, Te), and noble gases. 3.1w Elements can be differentiated by physical properties. ...
chapters 1-4
... H and He most abundant in space, O and Si in earth crust, O and C in human bodies. Atom – smallest building block; molecule – combination of two or more atoms. Can be an element or compound. ...
... H and He most abundant in space, O and Si in earth crust, O and C in human bodies. Atom – smallest building block; molecule – combination of two or more atoms. Can be an element or compound. ...
6.1 ATOMS, ELEMENTS, and COMPOUNDS
... • Compounds cannot be broken down into simpler compounds or elements by physical means. -can be broken down by chemical means into simpler compounds or into their original elements -H2O can be broken down into hydrogen gas and oxygen gas • Elements can undergo chemical reactions to combine with othe ...
... • Compounds cannot be broken down into simpler compounds or elements by physical means. -can be broken down by chemical means into simpler compounds or into their original elements -H2O can be broken down into hydrogen gas and oxygen gas • Elements can undergo chemical reactions to combine with othe ...
What is matter made of?
... Said that electrons orbit the nucleus along certain paths called energy levels or orbitals. Chemical properties are determined by the electrons in the outermost orbit. ...
... Said that electrons orbit the nucleus along certain paths called energy levels or orbitals. Chemical properties are determined by the electrons in the outermost orbit. ...
CHEM_Review - Kenston Local Schools
... single atom. Such a group of atoms is called a radical. if a radical Is used In a formula more than once, the radical is put in parentheses and the subscdpt appears outside the parentheses. When a subscript appears outside the parentheses, It indicates that all the demerits inside parentheses should ...
... single atom. Such a group of atoms is called a radical. if a radical Is used In a formula more than once, the radical is put in parentheses and the subscdpt appears outside the parentheses. When a subscript appears outside the parentheses, It indicates that all the demerits inside parentheses should ...
Unit 3 - Chemistry
... theories to explain his observations and came up with Dalton’s atomic theory. ...
... theories to explain his observations and came up with Dalton’s atomic theory. ...
Pre-Knowledge: Chemistry and Physics Vocabulary Atomic Number
... The sum of the number of neutrons and protons in the nucleus of an atom. Nucleus The small “core” of the atom, where most of its mass and all of its positive charge is concentrated. Except for ordinary hydrogen (which has only a proton), atomic nuclei consist of protons and neutrons. For this reason ...
... The sum of the number of neutrons and protons in the nucleus of an atom. Nucleus The small “core” of the atom, where most of its mass and all of its positive charge is concentrated. Except for ordinary hydrogen (which has only a proton), atomic nuclei consist of protons and neutrons. For this reason ...
ap chemistry chapter 8 bonding
... with a nonmetal • Ionic bonds form when an atom that loses electrons easily reacts with an atom that has a high affinity for electrons. The charged ions are held together by their mutual attraction. • Ionic bonds form because the ion pair has lower energy than the separated ions. All bonds form in o ...
... with a nonmetal • Ionic bonds form when an atom that loses electrons easily reacts with an atom that has a high affinity for electrons. The charged ions are held together by their mutual attraction. • Ionic bonds form because the ion pair has lower energy than the separated ions. All bonds form in o ...
Additional Chemistry
... Knowing the periodic table will help you first work out what bonding is taking place (ionic, covalent or metallic). ...
... Knowing the periodic table will help you first work out what bonding is taking place (ionic, covalent or metallic). ...
Chemical Bonds Study Guide Answer Key
... 10. metallic bonds- describes how metal atoms are held together in a solid 11. alloy- a combination of 2 or more elements, at least one is a metal 12. covalent bonds- bonds formed when nonmetals share electrons 13. polar bonds- covalent bonds that have a positive end and a negative end 14. dipoles- ...
... 10. metallic bonds- describes how metal atoms are held together in a solid 11. alloy- a combination of 2 or more elements, at least one is a metal 12. covalent bonds- bonds formed when nonmetals share electrons 13. polar bonds- covalent bonds that have a positive end and a negative end 14. dipoles- ...
Electronegativity

Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom or a functional group to attract electrons (or electron density) towards itself. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it. The term ""electronegativity"" was introduced by Jöns Jacob Berzelius in 1811,though the concept was known even before that and was studied by many chemists including Avogadro.In spite of its long history, an accurate scale of electronegativity had to wait till 1932, when Linus Pauling proposed an electronegativity scale, which depends on bond energies, as a development of valence bond theory. It has been shown to correlate with a number of other chemical properties. Electronegativity cannot be directly measured and must be calculated from other atomic or molecular properties. Several methods of calculation have been proposed, and although there may be small differences in the numerical values of the electronegativity, all methods show the same periodic trends between elements. The most commonly used method of calculation is that originally proposed by Linus Pauling. This gives a dimensionless quantity, commonly referred to as the Pauling scale, on a relative scale running from around 0.7 to 3.98 (hydrogen = 2.20). When other methods of calculation are used, it is conventional (although not obligatory) to quote the results on a scale that covers the same range of numerical values: this is known as an electronegativity in Pauling units. As it is usually calculated, electronegativity is not a property of an atom alone, but rather a property of an atom in a molecule. Properties of a free atom include ionization energy and electron affinity. It is to be expected that the electronegativity of an element will vary with its chemical environment, but it is usually considered to be a transferable property, that is to say that similar values will be valid in a variety of situations.On the most basic level, electronegativity is determined by factors like the nuclear charge (the more protons an atom has, the more ""pull"" it will have on electrons) and the number/location of other electrons present in the atomic shells (the more electrons an atom has, the farther from the nucleus the valence electrons will be, and as a result the less positive charge they will experience—both because of their increased distance from the nucleus, and because the other electrons in the lower energy core orbitals will act to shield the valence electrons from the positively charged nucleus).The opposite of electronegativity is electropositivity: a measure of an element's ability to donate electrons.Caesium is the least electronegative element in the periodic table (=0.79), while fluorine is most electronegative (=3.98). (Francium and caesium were originally assigned both assigned 0.7; caesium's value was later refined to 0.79, but no experimental data allows a similar refinement for francium. However, francium's ionization energy is known to be slightly higher than caesium's, in accordance with the relativistic stabilization of the 7s orbital, and this in turn implies that caesium is in fact more electronegative than francium.)