Observing Atomic Collapse Resonances in Artificial Nuclei on
... resonances are absent) was observed for charged impurities in graphene (14), but the observation of atomic collapse around supercritical impurities has remained elusive due to the difficulty of producing highly charged impurities. Here we report the observation of supercritical Coulomb behavior in a ...
... resonances are absent) was observed for charged impurities in graphene (14), but the observation of atomic collapse around supercritical impurities has remained elusive due to the difficulty of producing highly charged impurities. Here we report the observation of supercritical Coulomb behavior in a ...
atom interferometer - Center for Ultracold Atoms
... envelope of our atomic beam machine has been replaced by a series of 6-way crosses, achieving greater length, facilitating access to the equipment inside the chamber, and permitting the rapid reconfiguration of modular flanges which hold additional atom optical elements. The new apparatus is very st ...
... envelope of our atomic beam machine has been replaced by a series of 6-way crosses, achieving greater length, facilitating access to the equipment inside the chamber, and permitting the rapid reconfiguration of modular flanges which hold additional atom optical elements. The new apparatus is very st ...
Single-particle-sensitive imaging of freely propagating ultracold atoms
... Both the signal strength and the spatial resolution of the system depend on the geometrical and optical parameters of the light sheet. While this is obvious for the signal strength, the resolution’s dependence arises from the random scattering force fluctuations exerted on the atoms within the light ...
... Both the signal strength and the spatial resolution of the system depend on the geometrical and optical parameters of the light sheet. While this is obvious for the signal strength, the resolution’s dependence arises from the random scattering force fluctuations exerted on the atoms within the light ...
study guide - Mechanical and Nuclear Engineering
... 9. Explain the signi cance of wave-particle duality. 10. Describe how the Davisson-Germer electron scattering experiment con rmed the wave-nature of electrons. 11. Describe the basic idea used by Schrodinger to derive his famous wave equation. 12. Explain the important consequences of Schrodinger' ...
... 9. Explain the signi cance of wave-particle duality. 10. Describe how the Davisson-Germer electron scattering experiment con rmed the wave-nature of electrons. 11. Describe the basic idea used by Schrodinger to derive his famous wave equation. 12. Explain the important consequences of Schrodinger' ...
Spin Squeezing on an Atomic Clock Transition.
... Fig. 2. Measurement-induced pseudo-spin squeezing on an atomic clock transition. (a) Setup. A laser-cooled ensemble of 87 Rb atoms is loaded into a far-detuned optical dipole trap inside an optical resonator. A population difference N between hyperfine clock states |1⟩ , |2⟩ produces a resonator frequ ...
... Fig. 2. Measurement-induced pseudo-spin squeezing on an atomic clock transition. (a) Setup. A laser-cooled ensemble of 87 Rb atoms is loaded into a far-detuned optical dipole trap inside an optical resonator. A population difference N between hyperfine clock states |1⟩ , |2⟩ produces a resonator frequ ...
jyvaskla2 - School of Chemistry
... The partitioning in real space of molecules into atoms by using these interatomic surfaces is a fundamental, quantum mechanically rigorous, method. It allows properties to be calculated for proper open systems, where exchange, e.g. with charge may occur between atoms. The properties calculated by in ...
... The partitioning in real space of molecules into atoms by using these interatomic surfaces is a fundamental, quantum mechanically rigorous, method. It allows properties to be calculated for proper open systems, where exchange, e.g. with charge may occur between atoms. The properties calculated by in ...
test 3 practice
... ____ 36. What energy must be added or given off in a reaction where two hydrogen atoms and two neutrons are combined to form a helium atom? (Atomic masses for each: hydrogen, 1.007 825 u; neutron, 1.008 665 u; helium, 4.002 602 u; also, 1 u = 931.5 MeV/c2) a. 20.7 MeV added b. 20.7 MeV given off c. ...
... ____ 36. What energy must be added or given off in a reaction where two hydrogen atoms and two neutrons are combined to form a helium atom? (Atomic masses for each: hydrogen, 1.007 825 u; neutron, 1.008 665 u; helium, 4.002 602 u; also, 1 u = 931.5 MeV/c2) a. 20.7 MeV added b. 20.7 MeV given off c. ...
Slide 1
... In some chemical reactions, matter seems to be created. When a nail rusts. In other reactions, matter seems to disappear. When paper burns and apparently vanishes However, neither creation or destruction of matter occurs. Matter may undergo physical or chemical changes. ...
... In some chemical reactions, matter seems to be created. When a nail rusts. In other reactions, matter seems to disappear. When paper burns and apparently vanishes However, neither creation or destruction of matter occurs. Matter may undergo physical or chemical changes. ...
Chapter 8 - Cengage Learning
... are looking at the number of atoms or the mass of the atoms. In terms of numbers of atoms, ammonia is ¾ hydrogen (there are four atoms making up an ammonia molecule, and three of them are hydrogen). But it is often important to know the composition by mass of a compound. Chemists have instruments th ...
... are looking at the number of atoms or the mass of the atoms. In terms of numbers of atoms, ammonia is ¾ hydrogen (there are four atoms making up an ammonia molecule, and three of them are hydrogen). But it is often important to know the composition by mass of a compound. Chemists have instruments th ...
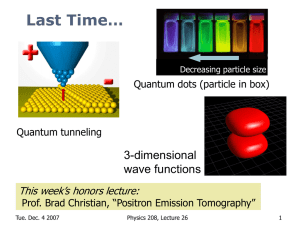
Historical Review of Quantum Mechanics
... begins with a short historical review of quantum mechanics. Then examines the properties of particles and waves, the Schrodinger equation, systems like the particle in a box, the harmonic oscillator. The lectures continue with a discussion of atomic structure & orbitals and Periodic Table. Continuou ...
... begins with a short historical review of quantum mechanics. Then examines the properties of particles and waves, the Schrodinger equation, systems like the particle in a box, the harmonic oscillator. The lectures continue with a discussion of atomic structure & orbitals and Periodic Table. Continuou ...
Quantum phenomena in gravitational field - AEgIS
... In the context of the general relativity theory, universality of a free fall is often referred to as the Weak Equivalence Principle (WEP). WEP is being tested with increasing sensitivity for macroscopic bodies. The best test so far confirms WEP to the accuracy of 2×10−13 (using a rotating torsion bal ...
... In the context of the general relativity theory, universality of a free fall is often referred to as the Weak Equivalence Principle (WEP). WEP is being tested with increasing sensitivity for macroscopic bodies. The best test so far confirms WEP to the accuracy of 2×10−13 (using a rotating torsion bal ...
Sample pages 2 PDF
... strong in comparison with other atomic interactions, as explained later in this chapter. Moreover, organic chemistry is not only present in living organisms, it is also involved in human health technologies like the development of new drugs or the study of materials, e.g. hypoalergenic materials, us ...
... strong in comparison with other atomic interactions, as explained later in this chapter. Moreover, organic chemistry is not only present in living organisms, it is also involved in human health technologies like the development of new drugs or the study of materials, e.g. hypoalergenic materials, us ...