Thermodynamic Cycles
... participants. Not for public distribution, delivery to, or reproduction by any third party without the prior agreement of the Academy. All other rights reserved. NOTICE: This information was prepared in connection with work sponsored by the Institute of Nuclear Power Operations (INPO). Neither INPO, ...
... participants. Not for public distribution, delivery to, or reproduction by any third party without the prior agreement of the Academy. All other rights reserved. NOTICE: This information was prepared in connection with work sponsored by the Institute of Nuclear Power Operations (INPO). Neither INPO, ...
basics of heat transfer
... and a warm canned drink left in a refrigerator cools down. This is accomplished by the transfer of energy from the warm medium to the cold one. The energy transfer is always from the higher temperature medium to the lower temperature one, and the energy transfer stops when the two mediums reach the ...
... and a warm canned drink left in a refrigerator cools down. This is accomplished by the transfer of energy from the warm medium to the cold one. The energy transfer is always from the higher temperature medium to the lower temperature one, and the energy transfer stops when the two mediums reach the ...
Simulation of the influence of preheating on stress - Purdue e-Pubs
... Because of kinds of reasons, defects, like crack, sand inclusion, pore and so on, often occurs during the productive of cast steel [1, 2]. This will worsen the properties of the products, even destroy it. Application of repair welding have been used to repair the defective cast steel in common [3, 4 ...
... Because of kinds of reasons, defects, like crack, sand inclusion, pore and so on, often occurs during the productive of cast steel [1, 2]. This will worsen the properties of the products, even destroy it. Application of repair welding have been used to repair the defective cast steel in common [3, 4 ...
Chp-1. Pure substances
... isothermal; & there is considerable increase in volume. At point E, all the water has vaporized & the state of steam at this point is called dry & saturated steam. The phase transformation from liquid to vapor is called vaporization. “The quantity of heat required for complete transformation of wate ...
... isothermal; & there is considerable increase in volume. At point E, all the water has vaporized & the state of steam at this point is called dry & saturated steam. The phase transformation from liquid to vapor is called vaporization. “The quantity of heat required for complete transformation of wate ...
Physics 231 Topic 14: Laws of Thermodynamics Wade Fisher
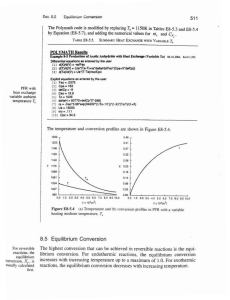
... e =1-Tcold/Thot carnot only!! In general: e < ecarnot ...
... e =1-Tcold/Thot carnot only!! In general: e < ecarnot ...
C2H5OH + 3 O2 → 2 CO2 + 3 H2O + HEAT Q = mc ∆T
... • the elements C & H2 have to be vaporized to gaseous atoms this is endothermic • then the bonds in C2H4 will be made – this is exothermic ...
... • the elements C & H2 have to be vaporized to gaseous atoms this is endothermic • then the bonds in C2H4 will be made – this is exothermic ...
TA INSTRUMENTS
... is achieved as with conventional isothermal experiments. During the temperature transition phases, heat flow is recorded to monitor how the sample is affected by the change in temperature. This mode is useful for the study of heat capacity and temperature dependence of chemical reactions. ...
... is achieved as with conventional isothermal experiments. During the temperature transition phases, heat flow is recorded to monitor how the sample is affected by the change in temperature. This mode is useful for the study of heat capacity and temperature dependence of chemical reactions. ...
Entropy
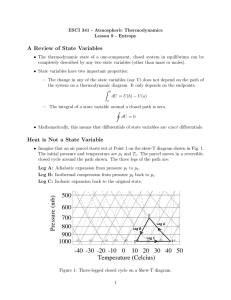
... • The thermodynamic state of a one-component, closed system in equilibrium can be completely described by any two state variables (other than mass or moles). • State variables have two important properties: – The change in any of the state variables (say U) does not depend on the path of the system ...
... • The thermodynamic state of a one-component, closed system in equilibrium can be completely described by any two state variables (other than mass or moles). • State variables have two important properties: – The change in any of the state variables (say U) does not depend on the path of the system ...
Ch 15) The Laws of Thermodynamics
... law of thermodynamics is a general statement of the law of conservation of energy. Note that the conservation of energy law was not able to be formulated until the 1800s, because it depended on the interpretation of heat as a transfer of energy. A given system does not “have” a certain amount of hea ...
... law of thermodynamics is a general statement of the law of conservation of energy. Note that the conservation of energy law was not able to be formulated until the 1800s, because it depended on the interpretation of heat as a transfer of energy. A given system does not “have” a certain amount of hea ...
First Law of Thermodynamics
... (w), less energy is transferred to the thermal surroundings (q). ...
... (w), less energy is transferred to the thermal surroundings (q). ...
Knowledge Check (Answer Key)
... such that 1 lbf of weight is generated by 1 lbm at the surface of the earth. This relationship is only true at the surface of the earth, however, where the acceleration due to gravity is exactly 32.17 ft/s2. Example: Using Newton’s second law, prove that 1 lbf is equivalent to 1 lbm on the earth's s ...
... such that 1 lbf of weight is generated by 1 lbm at the surface of the earth. This relationship is only true at the surface of the earth, however, where the acceleration due to gravity is exactly 32.17 ft/s2. Example: Using Newton’s second law, prove that 1 lbf is equivalent to 1 lbm on the earth's s ...
chapter i states of matter - myweb
... throughout. The components of homogeneous and heterogeneous mixtures can be separated and recovered as pure substances by means of physical methods. However, in the case of homogenous mixtures one has to be very careful with the recovery of pure solid substances. Consider for example the case of a s ...
... throughout. The components of homogeneous and heterogeneous mixtures can be separated and recovered as pure substances by means of physical methods. However, in the case of homogenous mixtures one has to be very careful with the recovery of pure solid substances. Consider for example the case of a s ...
Heat transfer

Heat transfer is the exchange of thermal energy between physical systems, depending on the temperature and pressure, by dissipating heat. The fundamental modes of heat transfer are conduction or diffusion, convection and radiation.Heat transfer always occurs from a region of high temperature to another region of lower temperature. Heat transfer changes the internal energy of both systems involved according to the First Law of Thermodynamics. The Second Law of Thermodynamics defines the concept of thermodynamic entropy, by measurable heat transfer.Thermal equilibrium is reached when all involved bodies and the surroundings reach the same temperature. Thermal expansion is the tendency of matter to change in volume in response to a change in temperature.