Bk3BP08EE
... The reaction of HBr with both trans- and cis-hex-3-ene results in the formation of the same product, 3-bromohexane, CH3CH2C*HBrCH2CH2CH3. Since carbon-3 is chiral, the product exhibits optical isomerism. In the reaction, H+ is first added to the carbon-4 to form a carbocation. The “+” charge is on c ...
... The reaction of HBr with both trans- and cis-hex-3-ene results in the formation of the same product, 3-bromohexane, CH3CH2C*HBrCH2CH2CH3. Since carbon-3 is chiral, the product exhibits optical isomerism. In the reaction, H+ is first added to the carbon-4 to form a carbocation. The “+” charge is on c ...
Chapter 4
... First prioritize the groups bonded to the two sp2 carbons If the higher priority group for each carbon is on the same side of the double bond, it is the Z isomer (for Zusammen, German for “together”) If the higher priority group for each carbon is on the opposite side of the double bond, it is the E ...
... First prioritize the groups bonded to the two sp2 carbons If the higher priority group for each carbon is on the same side of the double bond, it is the Z isomer (for Zusammen, German for “together”) If the higher priority group for each carbon is on the opposite side of the double bond, it is the E ...
Carbon-Carbon Bond Forming Reactions
... - electrophiles include halides, alcohols, olefins - cationic intermediate rearrangements of side chain possible - overalkylation is possible - deactivated benzenes and anilines do not react - must consider regioselectivity when using substituted benzenes directing effects: o/p or m (see handout) ...
... - electrophiles include halides, alcohols, olefins - cationic intermediate rearrangements of side chain possible - overalkylation is possible - deactivated benzenes and anilines do not react - must consider regioselectivity when using substituted benzenes directing effects: o/p or m (see handout) ...
Year 12 Unit 1b - Moulsham High School
... How would you separate the organic product of this reaction from the resulting mixture? ...
... How would you separate the organic product of this reaction from the resulting mixture? ...
Primary, secondary and tertiary haloalkanes and
... Primary, secondary and tertiary haloalkanes and alcohols QUESTION: Answer the following questions on primary, secondary and tertiary haloalkanes and alcohols 1) Molecule 1 below can be classified as a tertiary alcohol. Molecule 2 can be classified as a tertiary haloalkane. ...
... Primary, secondary and tertiary haloalkanes and alcohols QUESTION: Answer the following questions on primary, secondary and tertiary haloalkanes and alcohols 1) Molecule 1 below can be classified as a tertiary alcohol. Molecule 2 can be classified as a tertiary haloalkane. ...
Primary, secondary and tertiary haloalkanes and alcohols
... Primary, secondary and tertiary haloalkanes and alcohols QUESTION: Answer the following questions on primary, secondary and tertiary haloalkanes and alcohols 1) Molecule 1 below can be classified as a tertiary alcohol. Molecule 2 can be classified as a tertiary haloalkane. ...
... Primary, secondary and tertiary haloalkanes and alcohols QUESTION: Answer the following questions on primary, secondary and tertiary haloalkanes and alcohols 1) Molecule 1 below can be classified as a tertiary alcohol. Molecule 2 can be classified as a tertiary haloalkane. ...
Chapter 16.1
... Heat and Temperature • The energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter. • In one kind of calorimeter, known quantities of reactants are sealed in a reaction chamber that is immersed in a known quantity of water. • Energy given off by the reaction ...
... Heat and Temperature • The energy absorbed or released as heat in a chemical or physical change is measured in a calorimeter. • In one kind of calorimeter, known quantities of reactants are sealed in a reaction chamber that is immersed in a known quantity of water. • Energy given off by the reaction ...
Activity C14: Rate of a Chemical Reaction 1
... In this activity you will determine the effect of changes in concentration of the reactants on the rate of the chemical reaction. The reaction for this activity is the acidic reduction of the thiosulfate ion to sulfur and sulfur dioxide. The equation for the reaction is: S2O32-(aq) + 2 H+(aq) ====== ...
... In this activity you will determine the effect of changes in concentration of the reactants on the rate of the chemical reaction. The reaction for this activity is the acidic reduction of the thiosulfate ion to sulfur and sulfur dioxide. The equation for the reaction is: S2O32-(aq) + 2 H+(aq) ====== ...
Chapter 9 Reaction Energetics
... name, the enthalpy or heat of reaction , ΔH. Energy changes result because the potential energies of the products and reactants are different. The energy required for an endothermic reaction is used to convert reactants into products that are at higher potential energy (Figure 9.1a); i.e., endotherm ...
... name, the enthalpy or heat of reaction , ΔH. Energy changes result because the potential energies of the products and reactants are different. The energy required for an endothermic reaction is used to convert reactants into products that are at higher potential energy (Figure 9.1a); i.e., endotherm ...
Consider the following chemical equilibrium A B
... Adding H2 will cause the system to shift as to reduce the concentration of H2 to its original value. This will caus the system to produce more of NH3 b. Effect of volume and pressure If a system at equilibrium is disturbed by decreasing the volume (increasing the total pressure), the system respond ...
... Adding H2 will cause the system to shift as to reduce the concentration of H2 to its original value. This will caus the system to produce more of NH3 b. Effect of volume and pressure If a system at equilibrium is disturbed by decreasing the volume (increasing the total pressure), the system respond ...
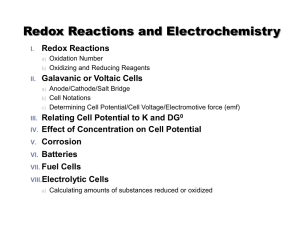
Redox Reactions
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred to the more electronegative atom. 3. The oxidation number of a transition metal ion is positive, but can vary in magnitude. 4. Nonmetals can have a variety of oxidation numbers,both positive ...
... The charge the atom would have in a molecule (or an ionic compound) if electrons were completely transferred to the more electronegative atom. 3. The oxidation number of a transition metal ion is positive, but can vary in magnitude. 4. Nonmetals can have a variety of oxidation numbers,both positive ...