Document
... Condensation polymers are those in which the molecular formula of the repeat unit of the polymer chain lacks certain atoms present in the monomer from which it is formed(or to which it can be degraded) for example, a polyester is formed by typical condensation reaction between bifunctional monomers, ...
... Condensation polymers are those in which the molecular formula of the repeat unit of the polymer chain lacks certain atoms present in the monomer from which it is formed(or to which it can be degraded) for example, a polyester is formed by typical condensation reaction between bifunctional monomers, ...
School of Chemistry and Physics Westville Campus, Durban
... Use HB Pencil and Tipp-ExTM are not allowed. This is Section A: Multiple Choice Questions, consisting of 18 pages. You are advised to spend not more than 2 hours on Section A. A periodic table and a data sheet are provided at the end of the Multiple Choice Questions ...
... Use HB Pencil and Tipp-ExTM are not allowed. This is Section A: Multiple Choice Questions, consisting of 18 pages. You are advised to spend not more than 2 hours on Section A. A periodic table and a data sheet are provided at the end of the Multiple Choice Questions ...
Name - Chemistry 302
... products side in the oxidation half-reaction and to the reactants side in the reduction halfreaction. This should always be true. 7. Make the number of electrons in both half-reactions equal by multiplying by coefficients. 8. Combine the two half-reactions. Combine any “like” terms and simplify! ...
... products side in the oxidation half-reaction and to the reactants side in the reduction halfreaction. This should always be true. 7. Make the number of electrons in both half-reactions equal by multiplying by coefficients. 8. Combine the two half-reactions. Combine any “like” terms and simplify! ...
File
... the change in energy (DE) in J, kJ, and kcal if the expanding gases do 515 J of work on the pistons, and and the system loses 407 J of heat to the cooling system. ...
... the change in energy (DE) in J, kJ, and kcal if the expanding gases do 515 J of work on the pistons, and and the system loses 407 J of heat to the cooling system. ...
aciee-2004-43-5442-palomo
... of reactant activation and reaction diastereo- and enantiocontrol, which may guide new developments. Yet, since some of these principles are too general and sometimes little supported, much effort will be needed to fully understand the basis of reactivity and selectivity. Much improvement is still n ...
... of reactant activation and reaction diastereo- and enantiocontrol, which may guide new developments. Yet, since some of these principles are too general and sometimes little supported, much effort will be needed to fully understand the basis of reactivity and selectivity. Much improvement is still n ...
chapter 1 - Revsworld
... Which of the following statements is/are correct? I. When heat energy flows from a system to the surroundings, we know that the temperature of the system is greater than that of the surroundings. II. Given the thermochemical equation 4NH3(g) + 5O2(g) ------> 4 NO(g) + 6H2O(g) H = -906 kJ, the therm ...
... Which of the following statements is/are correct? I. When heat energy flows from a system to the surroundings, we know that the temperature of the system is greater than that of the surroundings. II. Given the thermochemical equation 4NH3(g) + 5O2(g) ------> 4 NO(g) + 6H2O(g) H = -906 kJ, the therm ...
Thermodynamics
... adiabatically and reversibly to 5 atm pressure from an initial state of 20°C and 15 atm. What will be the final temperature and volume of the gas? What is the change in internal energy during this process? Assume a Cp of 8.58 cal/mole K (10 pts) 1 cal = 4.184 J ...
... adiabatically and reversibly to 5 atm pressure from an initial state of 20°C and 15 atm. What will be the final temperature and volume of the gas? What is the change in internal energy during this process? Assume a Cp of 8.58 cal/mole K (10 pts) 1 cal = 4.184 J ...
ANSWERS: Types of Reactions - Chemical Minds
... (accept ethanal, if its full structure is given) ii) CH3COONa or CH3COO- ...
... (accept ethanal, if its full structure is given) ii) CH3COONa or CH3COO- ...
Synthesis_of_Organometallic_Compounds
... A coordinatively unsaturated 16eruthenium(0) complex • Reduction of RuCl2(CO)2(PtBu2Me)2 with magnesium affords an isolable 16e ruthenium(0) complex Ru(CO)2(PtBu2Me)2. • Highly reactive toward hydrogen, acetylenes and phosphines to give coordinatively saturated complexes. ...
... A coordinatively unsaturated 16eruthenium(0) complex • Reduction of RuCl2(CO)2(PtBu2Me)2 with magnesium affords an isolable 16e ruthenium(0) complex Ru(CO)2(PtBu2Me)2. • Highly reactive toward hydrogen, acetylenes and phosphines to give coordinatively saturated complexes. ...
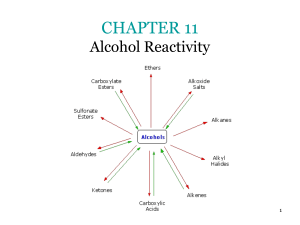
Organic Compounds Containing C, H and O
... Ans. i. a. Nitro (-NO2) group is an electron withdrawing whereas methoxy (-OCH3) group is electron releasing in nature. o-nitrophenol produces H+ ions easily but methoxyphenol does not. This is because o-nitrophenoxide ion is stabilised due to resonance. This is not true with o-methoxyphenoxide ion. ...
... Ans. i. a. Nitro (-NO2) group is an electron withdrawing whereas methoxy (-OCH3) group is electron releasing in nature. o-nitrophenol produces H+ ions easily but methoxyphenol does not. This is because o-nitrophenoxide ion is stabilised due to resonance. This is not true with o-methoxyphenoxide ion. ...