springs
... The basic motion of energy is stored work. A tankful of gas, a heavy truck moving at speed, and a charged automobile battery all possess energy. § The energy associated with a mass in motion is called kinetic energy § Energy an object possesses due to its condition or position is called potential ...
... The basic motion of energy is stored work. A tankful of gas, a heavy truck moving at speed, and a charged automobile battery all possess energy. § The energy associated with a mass in motion is called kinetic energy § Energy an object possesses due to its condition or position is called potential ...
CHM112 Lab – Heat of Neutralization – Grading Rubric
... The heat released by the reaction will be absorbed by the surroundings (aqueous solution). Coffee Cup Calorimetry will be employed to determine the amount of heat lost by the reaction and gained by the salt water solution. A calorimeter is simply a container used to measure the heat change. Cof ...
... The heat released by the reaction will be absorbed by the surroundings (aqueous solution). Coffee Cup Calorimetry will be employed to determine the amount of heat lost by the reaction and gained by the salt water solution. A calorimeter is simply a container used to measure the heat change. Cof ...
class-11thermodynamics
... 2. Microscopic property Property of individual atoms and molecules of a system at micro level atomic mass, molecular mass. ...
... 2. Microscopic property Property of individual atoms and molecules of a system at micro level atomic mass, molecular mass. ...
Chap8
... negative direction of the x axis when it is at x = 5.9 m, what is its speed when it passes through the origin? What are the answers to (c) (a) and (d) (b) if the potential energy of the system is taken to be -12 J when the object is at x = 0? (a) Number ...
... negative direction of the x axis when it is at x = 5.9 m, what is its speed when it passes through the origin? What are the answers to (c) (a) and (d) (b) if the potential energy of the system is taken to be -12 J when the object is at x = 0? (a) Number ...
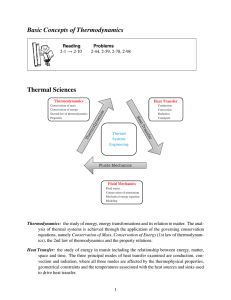
Basic Concepts of Thermodynamics Thermal Sciences
... Thermodynamics: the study of energy, energy transformations and its relation to matter. The analysis of thermal systems is achieved through the application of the governing conservation equations, namely Conservation of Mass, Conservation of Energy (1st law of thermodynamics), the 2nd law of thermod ...
... Thermodynamics: the study of energy, energy transformations and its relation to matter. The analysis of thermal systems is achieved through the application of the governing conservation equations, namely Conservation of Mass, Conservation of Energy (1st law of thermodynamics), the 2nd law of thermod ...
INTRODUCTION - WordPress.com
... This approach uses the statistical considerations and probability theory, where we deal with “average” ...
... This approach uses the statistical considerations and probability theory, where we deal with “average” ...
Elements and Atoms study guide Ch. 9.2 1. atom – the smallest
... a. vertical columns indicate elements that react in similar ways, chemically alike b. horizontal rows are called periods 12. matter – made up of elements in 3 states; liquid, solid, and gas 13. atomic theory – John Dalton concluded that gases are made up of solid particles that can be compressed tog ...
... a. vertical columns indicate elements that react in similar ways, chemically alike b. horizontal rows are called periods 12. matter – made up of elements in 3 states; liquid, solid, and gas 13. atomic theory – John Dalton concluded that gases are made up of solid particles that can be compressed tog ...
classical notions of heterogeneous freezing
... As temperature decreases, ice is formed in a super-cooled water droplet when the pressure of ice becomes less than the pressure of water, i.e., when a super-saturation with respect to ice appears. This brings about a change in Gibbs free energy per unit volume, Gv, between the water and ice phase. ...
... As temperature decreases, ice is formed in a super-cooled water droplet when the pressure of ice becomes less than the pressure of water, i.e., when a super-saturation with respect to ice appears. This brings about a change in Gibbs free energy per unit volume, Gv, between the water and ice phase. ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.