Physics 20 Lesson 27 Conservation of Energy
... The Law of Conservation of Energy states that the total amount of energy in a system remains constant. The energy may be transformed from one type to another, from kinetic to potential or kinetic to heat, but the total amount of energy is conserved. For example, imagine a skier starting from rest at ...
... The Law of Conservation of Energy states that the total amount of energy in a system remains constant. The energy may be transformed from one type to another, from kinetic to potential or kinetic to heat, but the total amount of energy is conserved. For example, imagine a skier starting from rest at ...
Change of state - Mrs. Coyle`s College Chemistry
... Extension of vapor pressure equilibrium… As you wait for boiling to occur, small bubbles of vapor appear. This pressure of the vapor is the vapor pressure of water at that temperature. As long as the vapor pressure is less than the atmospheric pressure, the bubbles collapse. Once they gain enough he ...
... Extension of vapor pressure equilibrium… As you wait for boiling to occur, small bubbles of vapor appear. This pressure of the vapor is the vapor pressure of water at that temperature. As long as the vapor pressure is less than the atmospheric pressure, the bubbles collapse. Once they gain enough he ...
Nature of Energy - Muskingum Valley Educational Service Center
... Standard: Physical Sciences: Students demonstrate an understanding of the composition of physical systems and the concepts and principles that describe and predict physical interactions and events in the natural world. This includes demonstrating an understanding of the structure and properties of m ...
... Standard: Physical Sciences: Students demonstrate an understanding of the composition of physical systems and the concepts and principles that describe and predict physical interactions and events in the natural world. This includes demonstrating an understanding of the structure and properties of m ...
CH08
... The gravitational force and the spring force are A B v0 called “conservative” because they can fk m fk m transfer energy from the kinetic energy of part x of the system to potential energy and vice versa. Frictional and drag forces on the other hand are called “nonconservative” for reasons that are ...
... The gravitational force and the spring force are A B v0 called “conservative” because they can fk m fk m transfer energy from the kinetic energy of part x of the system to potential energy and vice versa. Frictional and drag forces on the other hand are called “nonconservative” for reasons that are ...
What is Energy?
... Chemical energy in the fuel (gas or oil) is released as heat in the boiler when it burns with air. Heat converts water in boiler tubes to steam, which is then used to drive a turbine that converts energy into mechanical energy. Mechanical energy drives an alternator to produce electrical energy. ...
... Chemical energy in the fuel (gas or oil) is released as heat in the boiler when it burns with air. Heat converts water in boiler tubes to steam, which is then used to drive a turbine that converts energy into mechanical energy. Mechanical energy drives an alternator to produce electrical energy. ...
Period 6a Activity Solutions: Entropy
... vibrate the washers. This kinetic energy goes into increasing the internal energy of the cart. Since the elastic band cart has less kinetic energy after the collision, it does not move as far away from the wall. The cart with rigid rods rolls farther away from the wall because less of its initial ki ...
... vibrate the washers. This kinetic energy goes into increasing the internal energy of the cart. Since the elastic band cart has less kinetic energy after the collision, it does not move as far away from the wall. The cart with rigid rods rolls farther away from the wall because less of its initial ki ...
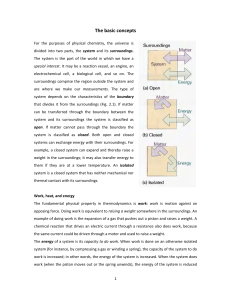
The basic concepts For the purposes of physical chemistry, the
... flows into the system with the lower temperature. If the temperature of either system at thermal equilibrium is raised infinitesimally, then energy flows out of the hotter system. Suppose a gas is confined by a piston and that the external pressure, Pex is set equal to the pressure, P, of the confi ...
... flows into the system with the lower temperature. If the temperature of either system at thermal equilibrium is raised infinitesimally, then energy flows out of the hotter system. Suppose a gas is confined by a piston and that the external pressure, Pex is set equal to the pressure, P, of the confi ...
Mechanical Energy and Simple Harmonic
... A block of mass m is attached to a spring with spring constant k is free to slide along a horizontal frictionless surface. At t = 0 the block-spring system is stretched an amount x0 > 0 from the equilibrium position and is released from rest. What is the x -component of the velocity of the block whe ...
... A block of mass m is attached to a spring with spring constant k is free to slide along a horizontal frictionless surface. At t = 0 the block-spring system is stretched an amount x0 > 0 from the equilibrium position and is released from rest. What is the x -component of the velocity of the block whe ...
Chapter 1 The Science of Physics
... conservation of momentum is NOT correct? a. Momentum is conserved for a system of objects pushing away from each other. b. Momentum is conserved when two or more interacting objects push away from each other. c. Momentum is not conserved for a system of objects in a head-on collision. d. The total m ...
... conservation of momentum is NOT correct? a. Momentum is conserved for a system of objects pushing away from each other. b. Momentum is conserved when two or more interacting objects push away from each other. c. Momentum is not conserved for a system of objects in a head-on collision. d. The total m ...
assignments
... words and the ramifications or interpretations of this law require a basic understanding of the law. Solution: The first law of thermodynamics can be stated as follows: the heat added to a system is equal to the increase in internal energy of the system plus the work done by the system. This is anot ...
... words and the ramifications or interpretations of this law require a basic understanding of the law. Solution: The first law of thermodynamics can be stated as follows: the heat added to a system is equal to the increase in internal energy of the system plus the work done by the system. This is anot ...
stable structure - Rothschild Science
... First- Let’s draw the Bohr model for Na and Cl How many valence electrons does each element have? What happens when the metal looses his ...
... First- Let’s draw the Bohr model for Na and Cl How many valence electrons does each element have? What happens when the metal looses his ...
THE STRUCTURE OF PHYSICAL CHEMISTRY
... which is an approximate description of (an approximate ‘equation of state’ for) the physical properties of gases (with p the pressure, V the volume, n the amount, R a universal constant, and T the temperature). We also encounter the laws of quantum mechanics, which summarize observations on the beha ...
... which is an approximate description of (an approximate ‘equation of state’ for) the physical properties of gases (with p the pressure, V the volume, n the amount, R a universal constant, and T the temperature). We also encounter the laws of quantum mechanics, which summarize observations on the beha ...
Heat transfer physics

Heat transfer physics describes the kinetics of energy storage, transport, and transformation by principal energy carriers: phonons (lattice vibration waves), electrons, fluid particles, and photons. Heat is energy stored in temperature-dependent motion of particles including electrons, atomic nuclei, individual atoms, and molecules. Heat is transferred to and from matter by the principal energy carriers. The state of energy stored within matter, or transported by the carriers, is described by a combination of classical and quantum statistical mechanics. The energy is also transformed (converted) among various carriers.The heat transfer processes (or kinetics) are governed by the rates at which various related physical phenomena occur, such as (for example) the rate of particle collisions in classical mechanics. These various states and kinetics determine the heat transfer, i.e., the net rate of energy storage or transport. Governing these process from the atomic level (atom or molecule length scale) to macroscale are the laws of thermodynamics, including conservation of energy.